|
|
|

|
|||||

|
|
Establishment Labs ESTA recently announced that it has submitted its Motiva breast implants to the FDA for approval in primary and revision breast reconstruction. The filing builds on the company’s September 2024 FDA approval for breast augmentation and marks a key step toward expanding Motiva’s U.S. addressable market beyond elective procedures.
From an investor standpoint, the submission highlights Establishment Labs’ strategy to leverage its existing clinical data and early commercial traction to move into a less innovation-heavy segment of breast care. With reconstruction volumes tied to mastectomy procedures and reimbursement-driven demand, a successful approval could create a more durable growth lever over time, even as near-term stock movement reflects broader market sentiment rather than the long-term opportunity.
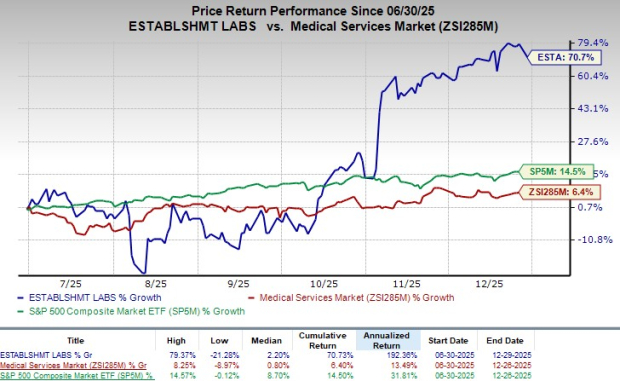
Following the announcement, ESTA shares lost 4.6% at yesterday’s market closing. However, shares of the company have surged 70.7% in the last six-month period compared with the industry’s 6.4% growth. The S&P 500 has gained 14.5% in the same time frame.
If approved, the reconstruction indication could meaningfully strengthen Establishment Labs' business by expanding Motiva into a clinically necessary, reimbursement-backed market with steadier procedure volumes than elective augmentation. This would allow ESTA to deepen relationships with hospitals and cancer centers, drive higher implant utilization alongside its already approved Motiva Flora tissue expander, and diversify revenue toward more predictable demand, supporting longer-term growth and margin stability.
ESTA currently has a market capitalization of $2.22 billion. The company projects an earnings growth of 54.84% for the next year.
The FDA submission is supported by clinical data from the Motiva U.S. IDE Study, which evaluated the safety and performance of Motiva implants in post-mastectomy reconstruction patients across multiple geographies. The study enrolled 274 patients in total, including both primary and revision reconstruction cases, providing a broad data set covering different clinical scenarios. The trial was conducted at 17 sites in the United States and one in Western Europe, reflecting a diverse surgical and patient population relevant to real-world reconstruction settings.
The filing includes Motiva SmoothSilk Round and Ergonomix implants, which are differentiated by their patented SmoothSilk surface technology aimed at enhancing biocompatibility and reducing inflammatory response. The Ergonomix implant is designed to dynamically adapt to body position, shifting between a teardrop profile when standing and a round profile when lying down, while the Round implant maintains consistent shape and upper-pole fullness regardless of position.
Since gaining FDA approval for breast augmentation in 2024, more than 60,000 Motiva implants have been sold in the U.S. market. In reconstruction, Establishment Labs already has a commercial presence through the Motiva Flora tissue expander, which received U.S. clearance in 2023 and is currently used in over 200 cancer centers, featuring MRI-conditional capability enabled by integrated radio-frequency port technology.
Per a report by Grand View Research, the global breast implants market size was estimated at $2.66 billion in 2024 and is projected to reach $5.16 billion by 2033, expanding at a CAGR of 7.7% from 2025 to 2033.
Market growth is attributed to the rising demand for aesthetic enhancement procedures, technological advancements in implant materials and increased awareness about body contouring solutions.
ESTA reported its third-quarter 2025 earnings in November, showcasing solid top-line growth and margin expansion. Total revenue rose to $53.8 million for the quarter ended Sept. 30, 2025, from $40.2 million in the prior-year period, reflecting stronger demand and improved pricing. Gross profit increased to $37.7 million, with gross margin expanding to 70.1% from 63.9% a year ago, driven mainly by favorable geographic mix and higher average selling prices.
Currently, ESTA carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the broader medical space are Intuitive Surgical ISRG, Medpace Holdings MEDP and Boston Scientific BSX.
Intuitive Surgical, sporting a Zacks Rank #1 (Strong Buy) at present, posted a third-quarter 2025 adjusted EPS of $2.40, beating the Zacks Consensus Estimate by 20.6%. Revenues of $2.51 billion topped the Zacks Consensus Estimate by 3.9%. You can see the complete list of today’s Zacks #1 Rank stocks here.
ISRG has an estimated long-term earnings growth rate of 15.7% compared with the industry’s 11.9% growth. The company’s earnings outpaced estimates in each of the trailing four quarters, the average surprise being 16.34%.
Medpace, currently carrying a Zacks Rank #2 (Buy), reported a third-quarter 2025 EPS of $3.86, which surpassed the Zacks Consensus Estimate by 10.29%. Revenues of $659.9 million beat the Zacks Consensus Estimate by 3.04%.
MEDP has an estimated earnings growth rate of 17.1% for 2025 compared with the industry’s 16.6% growth. The company’s earnings beat estimates in each of the trailing four quarters, the average surprise being 14.28%.
Boston Scientific, currently carrying a Zacks Rank #2, reported a third-quarter 2025 adjusted EPS of 75 cents, which surpassed the Zacks Consensus Estimate by 5.6%. Revenues of $5.07 billion topped the Zacks Consensus Estimate by 1.9%.
BSX has an estimated long-term earnings growth rate of 16.4% compared with the industry’s 13.5% growth. The company’s earnings beat estimates in each of the trailing four quarters, the average surprise being 7.36%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 5 hours | |
| 5 hours | |
| 6 hours | |
| 7 hours | |
| 7 hours | |
| 9 hours | |
| 10 hours | |
| 10 hours | |
| 11 hours | |
| 11 hours | |
| 12 hours | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite