|
|
|

|
|||||

|
|
Capricor Therapeutics CAPR recently provided a regulatory update on its Biologics License Application (BLA) for Deramiocel, following recent interactions with the FDA. After reviewing topline data from the Phase 3 HOPE-3 trial, the FDA has requested the full clinical study report (CSR) and supporting datasets as part of the ongoing BLA review. Importantly, the agency did not ask for any new clinical trials or additional patient data, signaling that the review remains focused on completing its assessment of existing results.
The company plans to submit the HOPE-3 CSR in February 2026, which management expects will address the issues outlined in the Complete Response Letter received last year and allow the FDA review process to continue, including the assignment of a new PDUFA date.
For investors, the update reinforces that Deramiocel remains on a defined regulatory path, with the FDA engagement centered on data completeness rather than clinical uncertainty.
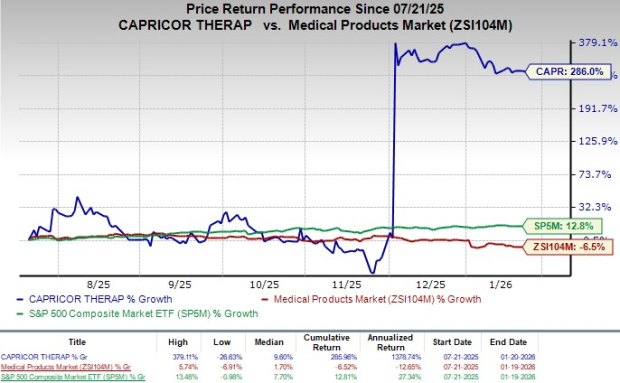
Following the announcement, the company's shares traded flat in Friday’s trading session. In the last six-month period, shares have gained 286% against the industry’s 6.5% decline. The S&P 500 has risen 12.8% over the same period.
In the long run, this update is constructive for CAPR because it keeps Deramiocel on a defined regulatory path without introducing new clinical trial risk. The FDA’s request for the HOPE-3 clinical study report, rather than additional trials, signals continued engagement and suggests the review is focused on data completeness, not efficacy doubts. If the February submission leads to a new PDUFA date, CAPR gains clearer approval visibility, strengthens Deramiocel’s credibility with regulators and partners, and moves closer to potential commercialization.
CAPR currently has a market capitalization of $1.11 billion.
The latest update from CAPR suggests the regulatory process for Deramiocel is moving in a measured and constructive direction. The FDA’s request for the full HOPE-3 clinical study report is a standard next step after reviewing topline data, especially in cases where the agency wants a deeper look at efficacy and safety details. Just as important for investors, the FDA did not request additional trials or new patient enrollment, reducing the risk of prolonged delays or higher development costs. Capricor expects to submit the CSR in February 2026, which should allow the BLA review to restart and lead to a new PDUFA action date.
Deramiocel itself remains a differentiated asset in Duchenne muscular dystrophy (DMD), particularly because it targets both cardiac and skeletal muscle function, two critical drivers of disease progression. The therapy’s mechanism, centered on cardiosphere-derived cells and their exosome-mediated immunomodulatory effects, positions it as a potentially disease-modifying option rather than a purely symptomatic treatment. With multiple regulatory designations already in hand, including RMAT and Orphan Drug status, Deramiocel benefits from an FDA framework designed to support faster review and closer regulatory engagement, which appears to be playing out in real time.
The Phase 3 HOPE-3 trial underpins this regulatory dialogue. The study enrolled both ambulatory and non-ambulatory DMD patients and was designed to capture clinically meaningful outcomes across disease stages. Management has highlighted statistically significant improvements in both muscle and cardiac measures, reinforcing the consistency seen in earlier studies.
Per a report by Grand View Research, the global DMD drugs market size was estimated at USD 3.47 billion in 2023 and is projected to reach USD 9.91 billion by 2030, expanding at a CAGR of 16.8% from 2024 to 2030.
This surge is caused by the increasing prevalence of road accidents and an aging population worldwide, which together are boosting demand for advanced trauma care solutions.
In December, CAPR provided updated results from its pivotal Phase 3 HOPE-3 trial, showing that Deramiocel met its primary endpoint on PUL v2.0 and the key secondary cardiac endpoint on LVEF, with both reaching statistical significance. All type 1 error–controlled secondary endpoints were also met, reinforcing the consistency of the data.
Importantly, the results demonstrated clinically meaningful improvements in both skeletal and cardiac function while maintaining a favorable safety and tolerability profile, supporting Deramiocel’s potential as a first-in-class therapy targeting Duchenne cardiomyopathy, the leading cause of mortality in DMD.Bottom of Form
Currently, CAPR carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the broader medical space are IDEXX Laboratories IDXX, Boston Scientific BSX and STERIS STE. Each stock presently carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for IDEXX’s 2025 earnings per share (EPS) have remained constant at $12.93 in the past 30 days. Shares of the company have risen 12.6% in the past year compared with the industry’s 11.1% growth. IDXX’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 7.1%. In the last reported quarter, it delivered an earnings surprise of 8.3%.
Boston Scientific shares have gained 2.9% in the past year. Estimates for the company’s 2025 EPS have remained constant at $3.04 in the past 30 days. BSX’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 7.4%. In the last reported quarter, it posted an earnings surprise of 5.6%.
STERIS shares have risen 9.1% in the past year. Estimates for the company’s 2025 EPS have increased by 2 cents to $10.23 in the past 30 days. STE’s earnings topped estimates in three of the trailing four quarters and matched on one occasion, delivering an average surprise of 2.6%. In the last reported quarter, it posted an earnings surprise of 2.6%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 6 hours | |
| 6 hours | |
| 11 hours | |
| 14 hours | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-18 | |
| Feb-16 | |
| Feb-15 | |
| Feb-15 | |
| Feb-15 | |
| Feb-13 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite