|
|
|

|
|||||

|
|
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Jan. 26, 2026 (GLOBE NEWSWIRE) -- MannKind Corporation (Nasdaq: MNKD), a biopharmaceutical company dedicated to transforming chronic disease care through innovative, patient-centric solutions for cardiometabolic and orphan lung diseases, today announced that the U.S. Food and Drug Administration (FDA) has approved an update to the Prescribing Information for Afrezza® (insulin human) Inhalation Powder, revising recommendations for the starting mealtime dosage when patients switch from subcutaneous mealtime insulin regimens.
“We expect that this label update will help support healthcare providers by providing clearer starting dose guidance when transitioning patients to inhaled insulin from subcutaneous mealtime insulin—whether injections or insulin pumps,” said Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Area Head, Diabetes at MannKind Corporation. “We believe this refinement to the label helps support appropriate initiation of therapy while reinforcing Afrezza’s established clinical profile.”
The updated labeling was supported by modeling data and in vivo results from the Dose Optimization study (see graph) and the INHALE-3 trial that demonstrated improved postprandial glucose outcomes following conversion to inhaled insulin using the now-approved conversion dose.
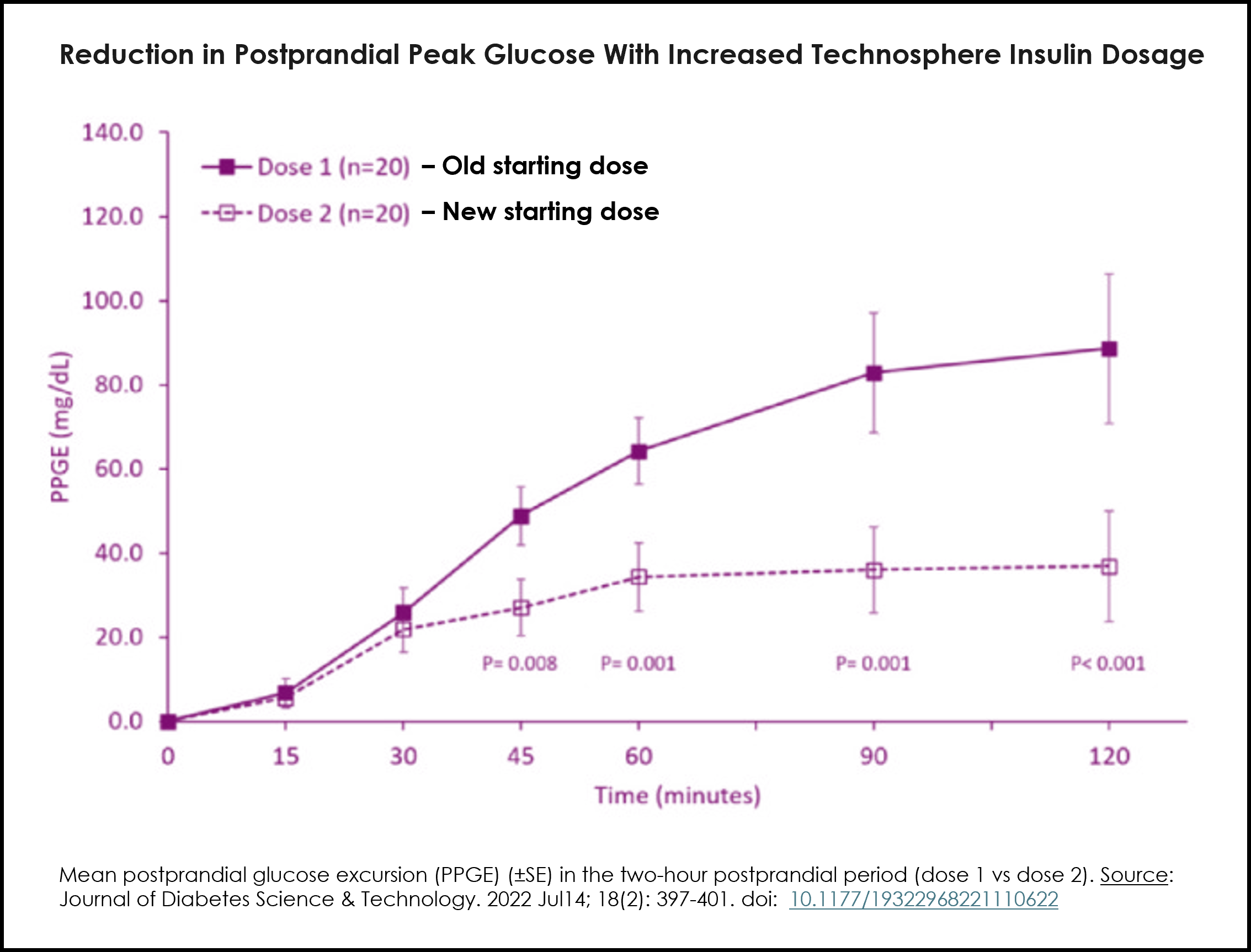
The revised dosing recommendations, provided in the table below, include recommended dose conversions from injected mealtime insulin or insulin pump bolus dosing to the appropriate mealtime dosage of Afrezza and are intended to support a clinically appropriate and safe transition for adult patients initiating Afrezza. Please see the Full Prescribing Information for additional details.
Table 1: Recommended Starting Mealtime Dosage of Afrezza when Switching from Other Mealtime Insulin Regimens
| Current Subcutaneous Mealtime Insulin Dosage | Starting Dosage of AFREZZA |
| Up to 3 units | 4 units |
| 4 to 5 units | 8 units |
| 6 to 7 units | 12 units |
| 8 or more units | 16 units |
About Afrezza
Afrezza® (pronounced uh-frezz-uh) Inhalation Powder is the only ultra rapid-acting inhaled insulin approved by the U.S. Food and Drug Administration to improve glycemic control in adult patients with diabetes mellitus. Administered at the beginning of meals using a small, portable inhaler, Afrezza delivers insulin via MannKind’s proprietary Technosphere® technology, enabling ultra-rapid absorption through the lungs. Afrezza has a fast onset of action and a short duration, more closely mirroring the body’s natural insulin response to meals.
INDICATION AND IMPORTANT SAFETY INFORMATION WITH WARNINGS
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
Afrezza is contraindicated: during episodes of hypoglycemia, in patients with chronic lung disease (such as asthma or COPD) because of the risk of acute bronchospasm, and in patients with previous severe hypersensitivity reaction to regular human insulin product or any of the inactive ingredients in Afrezza. Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with Afrezza.
In a study of patients with asthma whose bronchodilators were temporarily withheld for assessment, bronchoconstriction and wheezing following Afrezza dosing was reported. Before initiating therapy, evaluate all patients with a medical history, physical examination, and spirometry (FEV1) to identify potential underlying lung disease. Do not use in patients with chronic lung disease such as asthma or COPD.
Changes in an insulin regimen (e.g., insulin strength, manufacturer, injection site or type, or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. If clinically indicated, make any necessary changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. For patients with type 2 diabetes, dosage modifications of concomitant oral antidiabetic treatment may be needed.
Hypoglycemia is the most common adverse reaction associated with insulins, including Afrezza. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly, and symptoms may differ across patients and change over time in the same patient. Advise patients to recognize and manage hypoglycemia and self-monitor glucose. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of glucose monitoring is recommended.
Afrezza causes a decline in lung pulmonary function over time as measured by FEV1. In clinical trials excluding patients with chronic lung disease and lasting up to 2 years, Afrezza-treated patients experienced a small (40 mL) but greater FEV1 decline than comparator-treated patients. Assess pulmonary function with spirometry at baseline, after the first 6 months of therapy and annually thereafter even in the absence of pulmonary symptoms. In patients who have a decline of ≥20% in FEV1 from baseline, consider discontinuing Afrezza. Consider more frequent lung function assessment in patients with pulmonary symptoms, e.g., wheezing, bronchospasm, breathing difficulties, or persistent or recurring cough. If symptoms persist, discontinue Afrezza.
In clinical trials, 2 cases of lung cancer were observed in patients exposed to Afrezza while no cases were reported for the comparators. In both cases, a prior history of heavy tobacco use was identified as a risk factor for lung cancer. Two additional cases of lung cancer (squamous cell and lung blastoma) were reported in non-smokers exposed to Afrezza after the trial completion. These data are insufficient to determine whether Afrezza has an effect on lung or respiratory tract tumors. In patients with active lung cancer, a prior history of lung cancer, or in patients at risk of lung cancer, consider whether the benefits of Afrezza use outweigh this potential risk.
In clinical trials enrolling patients with type 1 diabetes, diabetic ketoacidosis (DKA) was more common in Afrezza-treated patients (0.43%; n=13) than in comparator-treated patients (0.14%; n=3). Patients with type 1 diabetes should always use Afrezza concomitantly with basal insulin. In patients at risk for DKA, such as those with an acute illness or infection, increase the frequency of glucose monitoring and consider discontinuing Afrezza and giving insulin using an alternate route of administration.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products. If hypersensitivity reactions occur, discontinue Afrezza, treat per standard of care and monitor until symptoms and signs resolve.
All insulin products, including Afrezza, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Observe these patients for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the TZD should be considered.
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
About MannKind
MannKind Corporation (Nasdaq: MNKD) is a biopharmaceutical company dedicated to transforming chronic disease care through innovative, patient-centric solutions. Focused on cardiometabolic and orphan lung diseases, we develop and commercialize treatments that address serious unmet medical needs, including diabetes, pulmonary hypertension, and fluid overload in heart failure and chronic kidney disease.
With deep expertise in drug-device combinations, MannKind aims to deliver therapies designed to fit seamlessly into daily life.
Learn more at mannkindcorp.com.
Forward-Looking Statements
Statements in this press release that are not statements of historical fact are forward-looking statements that involve risks and uncertainties. These statements include, without limitation, statements regarding clearer guidance regarding the initiation of insulin therapy. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind’s current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with developing and commercializing product candidates and other risks detailed in MannKind’s filings with the Securities and Exchange Commission (“SEC”), including under the “Risk Factors” heading of its most recently filed Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
AFREZZA, MANNKIND and TECHNOSPHERE are registered trademarks of MannKind Corporation.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/9c8efef3-3fac-4191-b791-45429abb7399
CONTACT: MannKind Contacts: Media Relations: Christie Iacangelo (818) 292-3500 [email protected] Investor Relations: Kate Miranda (781) 301-6869 [email protected]

| 1 hour | |
| 9 hours | |
| Feb-27 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 | |
| Feb-19 | |
| Feb-18 | |
| Feb-13 | |
| Feb-09 | |
| Jan-29 | |
| Jan-26 | |
| Jan-26 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite