|
|
|

|
|||||

|
|
Novo Nordisk NVO announced positive top-line data from a late-stage study in its global REIMAGINE clinical study program for a next-generation pipeline candidate, CagriSema, for treating adults with type II diabetes (T2D).
CagriSema is a fixed-dose combination of a long-acting amylin analogue, cagrilintide 2.4 mg and NVO’s blockbuster obesity drug, Wegovy (semaglutide 2.4 mg). The candidate demonstrated both superior HbA1c reduction and weight loss at week 68 compared with semaglutide, across all tested doses in the T2D study.
Novo Nordisk’s phase III REIMAGINE 2 study evaluated the 68-week efficacy and safety of once-weekly subcutaneous CagriSema in 2,728 adults with T2D inadequately controlled with metformin, with or without an SGLT2 inhibitor. The study compared two CagriSema doses (2.4 mg/2.4 mg and 1.0 mg/1.0 mg) against semaglutide (2.4 mg and 1.0 mg), cagrilintide (2.4 mg), and placebo. Roughly 40% of people were already using an SGLT2 inhibitor before the study initiated, providing insight into the therapy’s performance across commonly used treatment backgrounds.
Novo Nordisk’s phase III REIMAGINE 2 study data showed that CagriSema delivered superior glycemic control and weight loss compared with semaglutide alone in people with T2D. From a mean baseline HbA1c of 8.2%, patients treated with CagriSema 2.4 mg/2.4 mg achieved a 1.91 percentage-point reduction at 68 weeks compared with a 1.76 percentage-point reduction with semaglutide 2.4 mg, a statistically significant difference.
Weight-loss outcomes further differentiated CagriSema. From a mean baseline body weight of 101 kg, patients receiving the higher CagriSema dose achieved a superior 14.2% reduction in body weight after 68 weeks compared with 10.2% with semaglutide 2.4 mg. Notably, no weight-loss plateau was observed for CagriSema at week 68. In addition, 43% of patients achieved at least 15% weight loss, while 24% reached at least 20% weight loss, highlighting the depth of response.
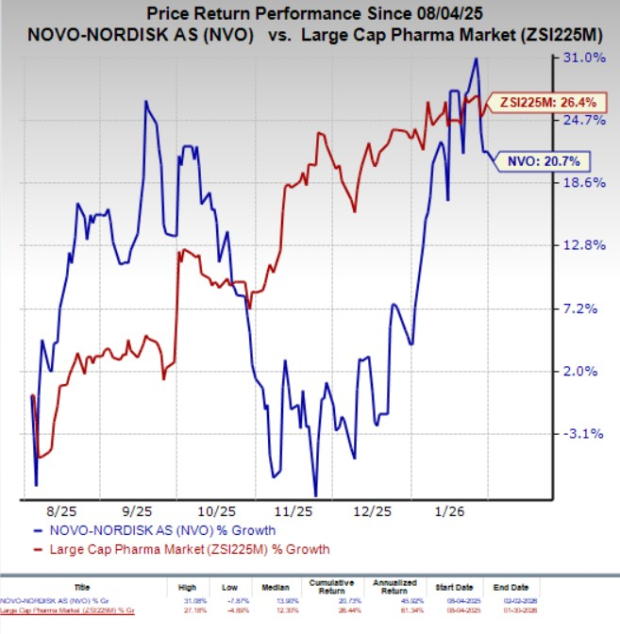
In the past six months, shares of Novo Nordisk have gained 20.7% compared with the industry’s 26.4% growth.
Results remained favorable under the treatment regimen estimand, which shows treatment effect regardless of treatment adherence. Under this analysis, CagriSema 2.4 mg/2.4 mg achieved a superior HbA1c reduction of 1.80 percentage points and weight loss of 12.9% compared with 1.68 percentage points HbA1c reduction and 9.2% weight loss, with semaglutide 2.4 mg after 68 weeks. Placebo-treated patients showed minimal change in HbA1c and limited weight loss.
From a safety perspective, CagriSema demonstrated a profile consistent with established incretin- and amylin-based therapies. The most reported adverse events were gastrointestinal in nature, were largely mild to moderate and tended to diminish over time. Overall, the data position CagriSema as a potentially differentiated therapy in T2D, with dual benefits in glucose control and clinically meaningful weight reduction.
Novo Nordisk is currently evaluating CagriSema as a treatment for adults with overweight or obesity in the phase III REDEFINE program and as a treatment for adults with T2D in the phase III REIMAGINE program.
Apart from the REIMAGINE 2 study, CagriSema is being evaluated in four other phase III studies for treating T2D. On the other hand, the REDEFINE program includes three phase III studies evaluating the candidate for obesity.
In the latest press release, Novo Nordisk reported that it intends to approach authorities to discuss the regulatory pathway for CagriSema in T2D following positive data readout from the phase III REIMAGINE 1 and REDEFINE 3 studies.
We remind the investors that in late 2025, Novo Nordisk filed a regulatory application seeking approval for CagriSema to reduce excess body weight and maintain weight reduction in the long term in adults with obesity or overweight. The FDA is expected to review the application in 2026. The candidate is intended to be used in conjunction with a reduced-calorie diet and increased physical activity in patients who have at least one weight-related comorbid condition.
The filing is based on statistically significant results from two late-stage studies, the 68-week phase III REDEFINE 1 study of CagriSema in obesity patients without diabetes and the 68-week phase III REDEFINE 2 study of the candidate in obesity patients with diabetes. If approved, NVO’s CagriSema would be the first injectable therapy to combine a GLP-1 RA with an amylin analogue.

Novo Nordisk A/S price-consensus-chart | Novo Nordisk A/S Quote
Novo Nordisk currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the biotech sector are Regeneron Pharmaceuticals REGN, Alkermes ALKS and Immunocore IMCR, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 60 days, estimates for Regeneron Pharmaceuticals’ 2026 EPS have increased from $41.80 to $44.19. Shares of REGN have surged 32.1% over the past six months.
Regeneron Pharmaceuticals’ earnings beat estimates in three of the trailing four quarters, missing the mark on the remaining occasion, delivering an average surprise of 22.92%.
Over the past 60 days, 2026 EPS estimates for Alkermes have increased from $1.54 to $1.91. Shares of ALKS have gained 30.6% over the past six months.
Alkermes’ earnings beat estimates in three of the trailing four quarters, missing on the remaining occasion, with the average surprise being 4.58%.
Over the past 60 days, estimates for Immunocore’s loss per share for 2026 have narrowed from 97 cents to 90 cents. IMCR stock has gained 1.6% over the past six months.
Immunocore’s earnings beat estimates in three of the trailing four quarters and missed in the remaining quarter, with the average surprise being 53.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 2 hours |
Stocks to Watch Monday Recap: Diamondback, Netflix, Novo Nordisk, Lilly
NVO -16.43%
The Wall Street Journal
|
| 2 hours | |
| 2 hours |
Hims & Hers Health Fourth-Quarter Sales Rise, Gives Soft First-Quarter Guidance
NVO -16.43%
The Wall Street Journal
|
| 3 hours | |
| 3 hours | |
| 3 hours | |
| 3 hours |
Novo Nordisk Dives As Next-Gen Obesity Drug Lags Eli Lilly's Kingpin
NVO -16.43%
Investor's Business Daily
|
| 4 hours | |
| 4 hours | |
| 4 hours | |
| 5 hours | |
| 5 hours | |
| 5 hours | |
| 5 hours | |
| 5 hours |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite