|
|
|

|
|||||

|
|
Johnson & Johnson JNJ announced that the FDA has granted approval to a key treatment, TAR-200 (gemcitabine intravesical system), for high-risk non-muscle invasive bladder cancer (NMIBC). This first-of-its-kind drug-releasing system, to be marketed by the name of Inlexzoh, is designed to give extended local delivery of a cancer medication into the bladder. The novel therapy is approved for treating certain patients with Bacillus Calmette-Guérin (BCG)-unresponsive NMIBC who have limited treatment options before possible surgical removal of the bladder. The approval of Inlexzoh is based on data from the phase IIb SunRISe-1 study.
J&J may launch Inlexzoh later this year, if approved. J&J believes that Inlexzoh is a transformational product that harnesses the company’s expertise in both Innovative Medicine and MedTech.
Inlexzoh’s approval shifts focus to J&J’s robust pipeline, which includes some key candidates as well as newly approved drugs with blockbuster potential. J&J has rapidly advanced its pipeline this year, attaining significant clinical and regulatory milestones that will help drive growth through the back half of the decade.
Nipocalimab, a FcRn blocker, was approved by the name of Imaavy in the United States in April this year for treating generalized myasthenia gravis, while an application is under review in the EU. It is also being evaluated in late-stage studies for various immune-mediated conditions like warm autoimmune hemolytic anemia, hemolytic disease of the fetus and newborn, and Sjogren’s disease and is in mid-stage studies for idiopathic inflammatory myopathy, rheumatoid arthritis and systemic lupus erythematosus. In fact, J&J believes that nipocalimab has a pipeline-in-a-product potential.
A new drug application (NDA) was filed for another key candidate, icotrokinra, for moderate-to-severe plaque psoriasis in July. Icotrokinra, an oral targeted peptide inhibitor of the IL-23 receptor, is also being evaluated in a phase II study for treating ulcerative colitis. J&J believes that, as a once-a-day pill, icotrokinra has the potential to set a new standard of care treatment for plaque psoriasis.
Three of J&J’s new cancer drugs are Carvykti, a BCMA CAR-T therapy for relapsed or refractory multiple myeloma, Tecvayli for relapsed or refractory multiple myeloma and Talvey, a novel bispecific therapy for heavily pretreated multiple myeloma. These drugs have also begun to contribute to top-line growth. Combined, they generated $1.3 billion in sales in the first half of 2025.
J&J’s latest acquisition of Intra-Cellular Therapies added antidepressant drug, Caplyta, to its neuroscience portfolio. Caplyta is already approved for the treatment of schizophrenia and is the only medicine approved for the treatment of depression in both bipolar 1 and 2. With a regulatory application under review, Caplyta is expected to be approved as an adjunctive treatment for major depressive disorder later in 2025.
J&J believes 10 of its new products/pipeline candidates in the Innovative Medicine segment have the potential to deliver peak sales of $5 billion, including Talvey, Tecvayli, Imaavy, Caplyta, Inlexzo and icotrokinra.
With Inlexzoh’s approval and a wave of promising late-stage candidates and recent product launches, J&J is well-positioned to sustain strong growth in its Innovative Medicine segment.
J&J enjoys a key presence in the oncology space. Its oncology sales now comprise around 40% of its pharmaceutical revenues, up 21.1% in the first half of 2025. While its older cancer drugs, Darzalex and Erleada, are key contributors to its top-line growth, new drugs like Carvykti, Tecvayli and Talvey hold the key for long-term growth. Other bigger players in the oncology space are AstraZeneca AZN, Merck MRK, Pfizer PFE and Bristol-Myers.
For AstraZeneca, oncology sales now comprise around 43% of total revenues. Sales in its oncology segment rose 16% in the first half of 2025. AstraZeneca’s strong oncology performance was driven by medicines such as Tagrisso, Lynparza, Imfinzi, Calquence and Enhertu (in partnership with Daiichi Sankyo).
Merck’s key oncology medicines are PD-L1 inhibitor Keytruda and PARP inhibitor Lynparza, which it markets in partnership with AstraZeneca. Keytruda, approved for several types of cancer, accounts for around 50% of Merck’s pharmaceutical sales. Keytruda’s sales rose 6.6% to $15.1 billion in the first half of 2025.
Oncology sales comprise more than 25% of Pfizer’s total revenues. Its oncology revenues grew 9% in the first half of 2025, driven by drugs like Xtandi, Lorbrena, the Braftovi-Mektovi combination and Padcev, which made up for declining sales of drugs like Ibrance.
Bristol-Myers’ key cancer drug is PD-L1 inhibitor, Opdivo, which accounts for around 20% of its total revenues. Opdivo’s sales rose 9% to $4.82 billion in the first half of 2025.
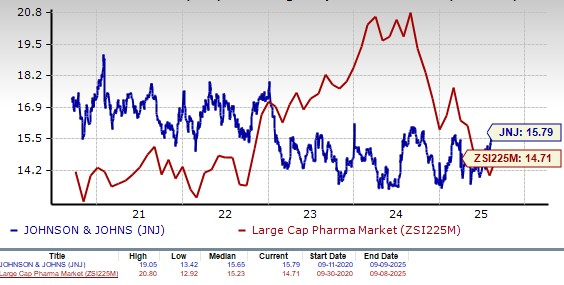
J&J’s shares have outperformed the industry year to date. The stock has risen 25.3% in the year-to-date period compared withan increase of 1.1% for the industry.

From a valuation standpoint, J&J is expensive. Going by the price/earnings ratio, the company’s shares currently trade at 15.79 forward earnings, higher than 14.71 for the industry. The stock is also trading above its five-year mean of 15.65.
The Zacks Consensus Estimate for 2025 earnings has risen from $10.64 per share to $10.86 per share for 2025 and from $11.09 per share to $11.37 per share over the past 60 days.

J&J has a Zacks Rank #2 (Buy) currently. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-27 | |
| Feb-27 |
These Stocks Lead Dow Jones In February. Hint: It's Not AI Companies.
MRK JNJ
Investor's Business Daily
|
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite