|
|
|

|
|||||

|
|
New Feature: See Wall Street analyst ratings directly on Finviz charts for deeper context into price action.
Biogen BIIB reported third-quarter 2025 adjusted earnings per share (EPS) of $4.81, which beat the Zacks Consensus Estimate of $3.89. Earnings rose 18% year over year.
Total revenues during the quarter came in at $2.53 billion, up 3% year over year on a reported basis and 2% on a constant-currency basis, driven by encouraging sales growth of new drugs. Sales of Biogen’s overall multiple sclerosis (MS) franchise also rose during the quarter, largely due to the strong demand for Vumerity, which more than offset the decline in Tecfidera sales. Revenues beat the Zacks Consensus Estimate of $2.34 billion.
Product sales in the quarter were $1.85 billion, up 4% year over year on a reported basis and 3% on a constant currency basis.
Revenues from anti-CD20 therapeutic programs rose 11% to $494 million. The revenues include royalties on sales of Roche’s RHHBY Ocrevus and Biogen’s share of Roche’s drugs, Rituxan, Gazyva and Lunsumio.
Contract manufacturing and royalty revenues declined 35% year over year to $151 million. Alzheimer’s collaboration revenues were $43 million compared with $19 million in the year-ago quarter.
Alzheimer’s collaboration revenues include Biogen’s 50% share of net product revenues and cost of sales (including royalties) from Alzheimer’s disease (AD) drug Leqembi (lecanemab), which has been developed in collaboration with Eisai. Eisai recorded nearly $121 million in global revenues from Leqembi sales in the third quarter, lower than $160 million in the previous quarter. The global sales in Q2 were higher due to a one-time shipment of about $35 million to China. The drug’s U.S. sales rose nearly 10% quarter over quarter to $69 million.
Leqembi has been launched in the United States, Japan, China, and some other countries and was approved in the European Union in April.
Biogen’s MS revenues totaled $1.06 billion, up 1% on a reported basis but stood relatively flat on a constant-currency basis, driven by a favorable gross-to-net adjustment.
Vumerity recorded nearly $215 million in sales, up around 36% year over year, driven by strong demand and favorable timing of shipments in the United States. Its sales beat the Zacks Consensus Estimate of $170 million and our model estimate of $173 million.
Tecfidera sales declined almost 28% to $168 million, owing to continued generic erosion of the drug in Europe. Despite this fall, the drug’s sales beat both the Zacks Consensus Estimate of $158 million and our model estimate of $163 million.
Tysabri sales rose 6% year over year to $432 million, driven by increased sales across all marketed regions. This drug’s sales beat the Zacks Consensus Estimate of $370 million and our model estimate of $347 million.
Combined interferon revenues (Avonex and Plegridy) rose 4% year over year during the quarter to $247 million.
Sales of Spinraza declined about 1% to $374 million. The figure beat the Zacks Consensus Estimate of $373 million and our estimate of $360 million.
Rare disease drug Skyclarys generated sales of $133 million, up 30% year over year, driven by continued demand growth and geographic expansion outside the United States. The drug’s U.S. sales fell 9% year over year due to the negative impact of Medicare discount dynamics.
New drug Qalsody recorded sales of over $26 million compared with $20 million in the previous quarter.
New drug Zurzuvae (for postpartum depression) recorded sales of over $55 million in the third quarter of 2025, up 19% on a sequential basis, driven by an increase in demand.
Biogen has a collaboration with Supernus Pharmaceuticals SUPN for Zurzuvae. Both companies equally share profits and losses for the drug’s commercialization in the United States. At the same time, outside U.S. markets, Biogen records product sales (excluding Japan, Taiwan and South Korea) and pays royalties to Supernus. Zurzuvae was approved for a similar indication in the EU last month.
Biosimilar revenues remained flat year over year at $197 million during the quarter.
Adjusted research and development (R&D) expenses declined 7% year over year to $432 million, driven by the company’s cost-saving initiatives under its “Fit for Growth” program and savings from the R&D portfolio prioritization efforts.
Adjusted selling, general and administrative (SG&A) expenses rose 6% to $592 million due to higher costs to support the new product launches, partially offset by cost savings under the “Fit for Growth” program.
The company slightly raised its sales guidance for the full year. It now expects the metric to be approximately flat or rise by 1% in constant currency terms versus the 2024 level, reflecting an improvement from the prior expectation of nearly flat growth.
Biogen believes that its domestic MS sales were better than expected in the first nine months of 2025, driven by demand for Vumerity, as well as favorable gross-to-net adjustments and favorable inventory timing. However, the franchise’s sales are expected to decline in Q4 due to increased competitive pressure in ex-U.S. territories.
However, Biogen lowered its adjusted EPS guidance from $15.50-$16.00 to $14.50-$15.00. While this updated figure reflects a ~$0.25 benefit from stronger business performance, it was offset by an expected ~$1.25 impact from business development transactions expected to close in Q4.
Combined R&D and SG&A costs are expected to be around $1.1 billion in the fourth quarter of 2025.
Alongside the results, Biogen announced that it resubmitted the regulatory filing seeking the FDA’s approval for a higher dose of Spinraza (nusinersen). A final decision is expected by April 3, 2026.
The initial filing received a complete response letter (CRL) from the agency last month. Per this CRL, the FDA requested that an update to the technical information be added to the Chemistry, Manufacturing and Controls (CMC) section of the filing. The letter did not cite any deficiencies related to the clinical data of the high-dose regimen.
Biogen also announced that it has completed enrolment in both late-stage studies evaluating litifilimab for systemic lupus erythematosus (SLE). Data readouts from the studies are expected in the second half of 2026.
Biogen reported better-than-expected Q3 results, driven by robust sales growth of new drugs like Leqembi, Skyclarys and Zurzuvae. Combined, sales of these launch products surged 67% year over year. Key MS products — Tecfidera, Vumerity and Tysabri — also surpassed expectations.
Backed by this strong performance, Biogen raised its total revenue guidance for 2025. However, investor sentiment was negatively impacted following the company’s decision to lower its EPS guidance to reflect expected R&D and deal-related costs tied to its pending business development transactions. These include the Alcyone Therapeutics acquisition (expected to close by this year-end) and licensing deal for Vanqua Bio’s preclinical oral C5aR1 antagonist drug. This likely explains the stock’s 3% premarket dip today.
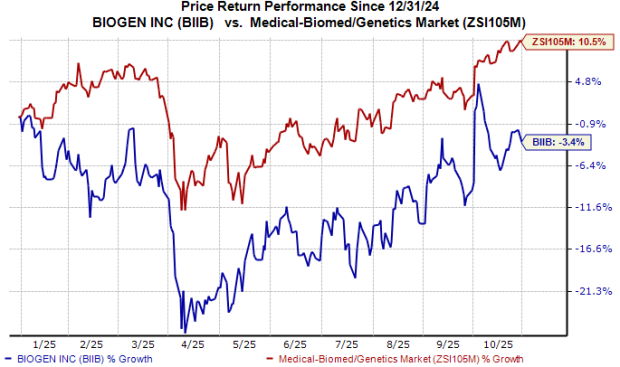
So far this year, the stock has declined over 3% against the industry’s nearly 11% growth.
Though Biogen’s MS drugs and Spinraza are seeing rising competitive pressure, the company’s new products have the potential to revive growth in the long term. BIIB is making significant progress toward building a multi-franchise portfolio through both internal development and collaborations.
The company believes that its four key pipeline products — litifilimab, dapirolizumab pegol (for SLE), felzartamab (for kidney-related diseases) and BIIB080 (for AD) — have $14 billion of peak revenue potential.

Biogen Inc. price | Biogen Inc. Quote
Biogen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-20 | |
| Feb-19 | |
| Feb-18 | |
| Feb-17 | |
| Feb-16 | |
| Feb-16 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-12 | |
| Feb-12 | |
| Feb-11 | |
| Feb-11 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite