|
|
|

|
|||||

|
|
Abbott Laboratories ABT recently received FDA approval for its Volt PFA System to treat patients battling atrial fibrillation (AFib). The company is expected to begin commercial PFA cases in the United States and continue expanding its site footprint in the European Union following the Volt CE Mark approval earlier this year.
Following the news, shares of ABT slightly dipped 0.2% yesterday. Over the past year, shares of the company have climbed 9.5% compared with the industry’s 0.5% growth.
On a positive note, Abbott’s accelerated investments in the R&D pipeline are yielding positive results. Hence, we expect the market sentiment toward ABT stock to turn positive surrounding the latest approval.
ABT currently has a market capitalization of $218.16 billion. The company’s earnings yield of 4.1% surpasses the industry’s 0.1%. ABT delivered an average earnings beat of 0.7% in the trailing four quarters.
FDA approval for the Volt PFA System was secured based on strong results from Abbott's VOLT-AF IDE study, a clinical trial of 392 patients conducted at 40 centers in the United States, Europe, Canada and Australia. The data showed that the Volt PFA System demonstrated clinically meaningful performance in both safety and effectiveness in two different patient groups — people battling paroxysmal atrial fibrillation (PAF) as well as persistent AFib (PersAF).
The Volt PFA System is built upon the company's leading electrophysiology portfolio by providing an all-in-one product that allows physicians to safely map, pace and ablate with the same catheter. Volt PFA System was designed to integrate with Abbott's EnSite X EP System by providing physicians with accurate 3D cardiac mapping and fewer catheter exchanges during an ablation.
Volt's proprietary balloon-in-basket design features multiple handling options for ease of use and allows for efficient energy transfer directly to the targeted tissue to stop the heart's erratic signals. The Volt PFA System is designed to deliver precise, targeted energy during ablation, which helps achieve durable lesions with fewer pulses. This level of accuracy supports effective first-time procedures, reducing the likelihood of repeat ablations and minimizing the risk of complications.
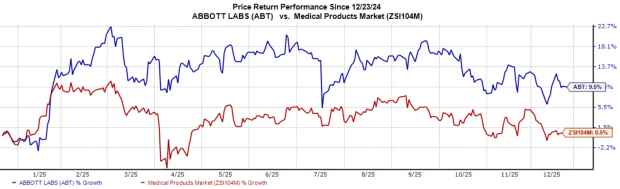
Image Source: Zacks Investment Research
Patients who undergo a minimally invasive ablation procedure with the Volt PFA Catheter can be placed under conscious sedation instead of general anesthesia, which is a significant benefit for patients where anesthesia is a barrier to performing ablations. The Volt PFA System also reduces exposure to radiation (fluoroscopy) and limits the breakdown of red blood cells (hemolysis).
According to reports published by CDC, nearly 12 million people in the United States over the age of 65 have AFib, a number expected to double over the next 20 years. People living with AFib face a fivefold increased risk of stroke, and the condition has been a contributing cause of death for more than two decades in the United States. When medication and other treatment options fail to work, many patients rely on a minimally invasive cardiac ablation procedure to effectively treat the condition by stopping irregular heart rhythms.
Abbott recently announced that Lingo, its first over-the-counter biowearable continuous glucose monitor system (CGM), can now be used with Android devices. Previously available only on Apple iOS, this latest development significantly expands the addressable user base and allows millions of users to track real-time glucose levels.
Abbott currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are BrightSpring Health Services BTSG, Illumina ILMN and Insulet PODD. While BrightSpring and Illumina sport a Zacks Rank #1 (Strong Buy) each, Insulet carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Estimates for BrightSpring Health Services’ 2025 EPS have increased 5.7% in the past 30 days. Shares of the company have surged 94.8% in the past year compared with the industry’s 6.7% growth. BTSG’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 45.1%. In the last reported quarter, it delivered an earnings surprise of 11.1%.
Illumina shares have lost 5.7% over the past year. Estimates for the company’s 2025 EPS have jumped 0.9% to $4.71 in the past 30 days. ILMN’s earnings beat estimates in three of the trailing four quarters and missed on one occasion, delivering an average surprise of 6.7%. In the last reported quarter, it posted an earnings surprise of 15.5%.
Insulet shares have risen 10.7% over the past year. Estimates for the company’s 2025 EPS have increased 6.5% to $4.89 in the past 60 days. PODD’s earnings topped estimates in each of the trailing four quarters, delivering an average surprise of 17.8%. In the last reported quarter, it posted an earnings surprise of 9.7%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 6 hours | |
| 8 hours | |
| 9 hours | |
| 9 hours | |
| 10 hours | |
| 11 hours | |
| 13 hours | |
| 13 hours | |
| 13 hours | |
| 13 hours | |
| 14 hours | |
| 15 hours | |
| 15 hours | |
| 15 hours | |
| 15 hours |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite