|
|
|

|
|||||

|
|
CorMedix CRMD has been riding on the success of its lead product, DefenCath (taurolidine plus heparin), which was approved by the FDA in 2023 as the first and only antimicrobial catheter lock solution in the United States. The product remains the company’s primary source of revenue. DefenCath is indicated to reduce the incidence of catheter-related bloodstream infections (CRBSIs) in adult patients with kidney failure who receive chronic hemodialysis through a central venous catheter. The product was launched in 2024 in both hospital inpatient and outpatient hemodialysis settings and has witnessed strong market adoption since then.
In the first nine months of 2025, DefenCath recorded $167.6 million in net sales, reflecting strong uptake in its early commercial journey. Importantly, DefenCath holds a unique market position as the only FDA-approved therapy for a niche condition, supported by patent protection through 2033. CorMedix is also planning future potential label expansion of DefenCath into total parenteral nutrition to increase its customer base.
In addition to DefenCath, CorMedix is benefiting from its $300 million acquisition of Melinta Therapeutics, which aims to diversify revenues and strengthen its presence in hospital acute care and infectious disease markets. Reflecting the growing momentum with DefenCath and early Melinta portfolio contributions, the company recently reported preliminary unaudited 2025 pro forma net revenues of about $400 million, which were within its 2025 guidance ($390 million to $410 million).
However, CorMedix’s financial outlook for 2026 seemed to have tempered investor expectations around DefenCath’s growth trajectory. Management introduced full-year 2026 revenue guidance of $300-$320 million, including $150-$170 million from DefenCath.
Notably, DefenCath sales are weighted toward the first half of 2026, with only modest utilization gains offsetting ongoing pricing pressure. Importantly, CorMedix projected 2027 DefenCath revenues of $100-$140 million based on higher net selling prices compared to 2026. DefenCath guidance for 2026 and 2027 assumes flat usage among existing customers and excludes any benefit from new accounts, Medicare Advantage contracting, or reimbursement changes — signaling a more conservative, slower-growth outlook than many investors had anticipated. CorMedix anticipates adjusted EBITDA to be in the range of $100 million to $125 million for full-year 2026.
Though DefenCath is expected to have been a key top-line driver for CorMedix in the fourth quarter of 2025, it remains to be seen how the recent outlook impacts DefenCath's long-term growth and customer adoption in the face of this bearish sentiment. Additionally, competition from major players like Pfizer PFE, Amphastar Pharmaceuticals AMPH, B. Braun, Baxter, and Fresenius Kabi USA that already market heparin for multiple uses remains a concern.
DefenCath combines taurolidine, an antimicrobial agent, with heparin in a fixed-dose formulation, tailored for a specific subset of kidney failure patients. Although CorMedix currently holds a first-mover advantage in the United States with DefenCath, the broader competitive environment still poses risks.
Pfizer, which sells Heparin Sodium Injection across multiple indications such as dialysis, surgery and thrombosis, could leverage its global scale and expertise to enter the CRBSI prevention market. Amphastar Pharmaceuticals, with end-to-end control over enoxaparin production, also has the efficiency and technical capabilities to pursue similar opportunities.
Given their stronger pipelines, larger manufacturing capabilities and greater financial resources, these companies could quickly become significant competitors if they decide to target CRBSI-related indications — a move that could challenge CorMedix’s market advantage and affect its long-term growth outlook.
Also, if either Pfizer or Amphastar Pharmaceuticals expands its anticoagulant portfolio into catheter-related infection prevention, CorMedix could encounter significant competitive pressure within its primary therapeutic space.
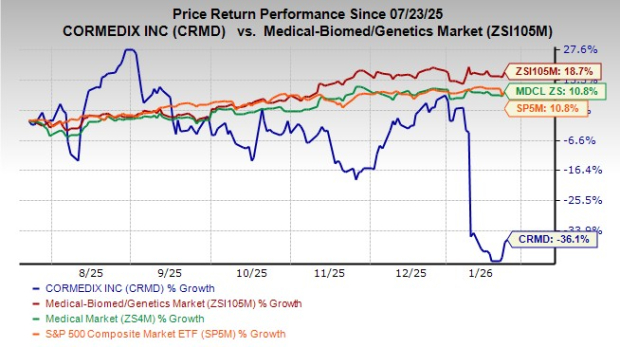
Shares of CorMedix have plunged 36.1% in the past six months against the industry’s growth of 18.7%. The stock has also underperformed the sector and the S&P 500 index during the same time frame, as seen in the chart below.
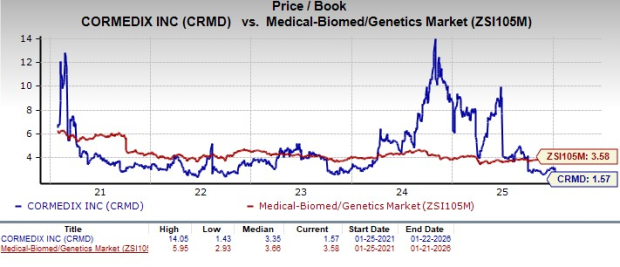
From a valuation standpoint, CorMedix is trading at a discount to the industry. Going by the price/book ratio, the company’s shares currently trade at 1.57, lower than 3.58 for the industry. The stock is also trading below its five-year mean of 3.35.
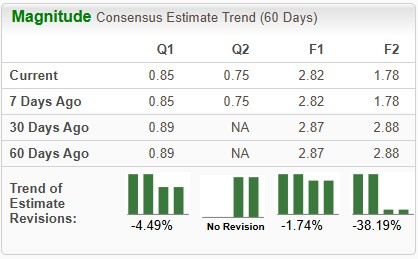
Estimates for CorMedix’s 2025 earnings have decreased from $2.87 to $2.82 per share in the past 60 days, while estimates for 2026 earnings have declined from $2.88 to $1.78 over the same timeframe.
CorMedix currently carries a Zacks Rank #5 (Strong Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 2 hours | |
| 3 hours | |
| 3 hours | |
| 4 hours | |
| 4 hours | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 | |
| Mar-09 |
Roche Shares Fall After Breast-Cancer Treatment Misses Goal in Late-Stage Study
PFE
The Wall Street Journal
|
| Mar-09 | |
| Mar-08 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about Finviz Elite