|
|
|

|
|||||

|
|
Alkermes ALKS announced positive top-line data from the phase II Vibrance-1 study, which evaluated its novel, investigational, and oral orexin 2 receptor agonist, alixorexton (formerly ALKS 2680), for treating patients with narcolepsy type 1 (NT1).
In the Vibrance-1 study, patients were randomized to receive alixorexton (4 mg, 6 mg or 8 mg) or placebo to be taken once daily for six weeks.
Data from the study showed that treatment with alixorexton across all doses led to a statistically significant, clinically meaningful and dose-dependent improvement from baseline versus placebo in wakefulness on the Maintenance of Wakefulness Test (MWT) — the study’s primary endpoint.
Treatment with alixorexton across all doses demonstrated statistically significant and clinically meaningful improvements from baseline in excessive daytime sleepiness versus placebo at week six on the Epworth Sleepiness Scale — a key secondary endpoint of the Vibrance-1 study.
Although all three doses of alixorexton led to improvements in weekly cataplexy rates — also a key secondary endpoint of the study — only the 6 mg dose achieved statistical significance. This might have hurt investor sentiment and caused the stock to decline 8.8% yesterday.
Also, treatment with once-daily alixorexton led to robust and clinically meaningful improvements in patient-reported outcomes for excessive daytime sleepiness, fatigue and cognition compared to placebo.
Treatment with alixorexton across all doses was generally safe and well-tolerated.
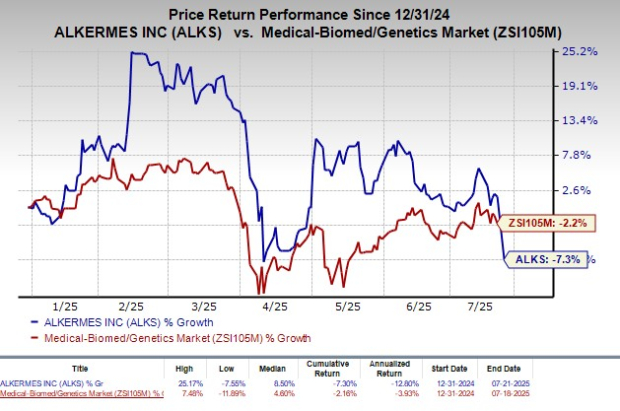
Shares of Alkermes have declined 7.3% so far this year compared with the industry’s decrease of 2.2%.
Building on the overall success of the Vibrance-1 study, management is planning to initiate a global phase III program for alixorexton in patients with NT1.
NT1 is a chronic sleep disorder causing excessive daytime sleepiness and sudden muscle weakness called cataplexy due to orexin deficiency.
We note that orexin agonists like alixorexton directly target the brain’s orexin system — the root cause of NT1. Unlike other available drugs that only fight sleepiness, orexin agonists may also prevent cataplexy, offering a more natural and complete treatment approach. This can make them a promising and transformative treatment in narcolepsy care.
The detailed safety and efficacy data from the phase II Vibrance-1 study are expected to be presented at an upcoming scientific conference.
Besides NT1, alixorexton is also being studied for the treatment of narcolepsy type 2 (“NT2”) and idiopathic hypersomnia (“IH”).
The phase II Vibrance-2 study is evaluating the safety and efficacy of alixorexton versus placebo in adults with NT2. Enrollment in this study is expected to be completed shortly, with data from the same expected during fall.
In April 2025, the company initiated the phase II Vibrance-3 study, evaluating the safety and efficacy of alixorexton in adults with idiopathic hypersomnia, a rare, chronic and neurological sleep disorder.
Per management, if successfully developed and upon potential approval, alixorexton can serve an area of high unmet medical need in the treatment of NT1 and NT2 as well as IH.
However, upon potential approval, alixorexton is likely to face competition from Axsome’s AXSM Sunosi (solriamfetol), which is presently marketed in the United States for the treatment of narcolepsy.
Several label expansion studies on solriamfetol are also currently underway.
Axsome acquired the U.S. rights for Sunosi from Jazz Pharmaceuticals JAZZ in May 2022. AXSM began selling Sunosi in the U.S. market in May 2022.
JAZZ received approval for Sunosi as a treatment for narcolepsy in 2019.
Jazz’s other sleep disorder drugs, Xyrem and Xywav, also hold a strong market share.

Alkermes plc price | Alkermes plc Quote
Alkermes currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the biotech sector is Arvinas ARVN, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Arvinas’ 2025 loss per share have narrowed from $1.60 to $1.51. Loss per share estimates for 2026 have narrowed from $3.28 to $2.98 during the same period. ARVN stock has plunged 60.7% year to date.
Arvinas’ earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 82.09%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 17 hours | |
| Feb-28 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite