|
|
|

|
|||||

|
|
Novavax NVAX reported second-quarter 2025 earnings per share (EPS) of 62 cents against the Zacks Consensus Estimate of a loss of seven cents per share. However, the reported figure declined 37% year over year.
Quarterly revenues totaled $239 million, down 42% year over year. Yet, the metric beat the Zacks Consensus Estimate of $118 million.
Novavax recorded $11 million in product sales compared with $23 million in the year-ago period. The reported figure includes a net reversal of $2 million in sales of its sole marketed product, the COVID vaccine Nuvaxovid, and $13 million from supply sales, which comprise finished product, adjuvant and other materials sold to licensed partners.
Licensing, royalties and other revenues totaled $228.5 million, which includes $175 million recognized as a milestone payment from partner Sanofi SNY. This payment was triggered after the company received full approval from the FDA for Nuvaxovid in May. Yet, the metric declined 42% year over year, due to the receipt of $391 million from SNY as an upfront payment associated with the collaboration agreement signed last year.
Starting this year, Sanofi acquired exclusive rights to market Nuvaxovid globally, except in certain territories where Novavax maintains existing partnership agreements. Alongside the latest earnings release, NVAX announced that it completed the transition of the vaccine’s commercial leadership to SNY for the 2025-2026 vaccination season.
Shares of Novavax rose nearly 13% yesterday, attributable to this transition. A pharma giant like Sanofi, which already has a well-built distribution and commercial supply chain across the globe, is likely better placed to market Nuvaxovid than the company for the current vaccination season.
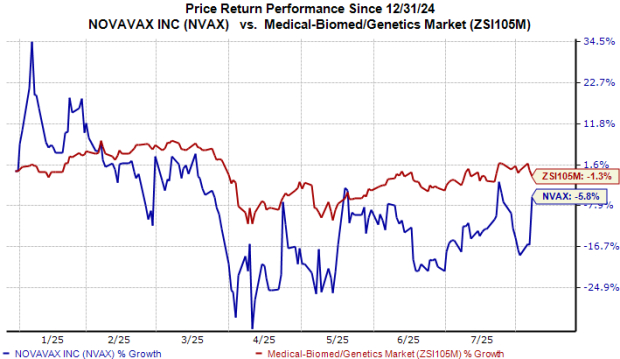
Year to date, the stock fell 6% compared with the industry’s 1% decline.
In the reported quarter, research and development (R&D) expenses totaled $79 million, down 26% year over year. The downside was caused by a reduction in overall expenditures relating to COVID vaccine development.
Selling, general and administrative (SG&A) expenses decreased 57% year over year to $44 million. This downtick was due to several factors, including the transition of lead commercial activities to Sanofi, the elimination of commercial infrastructure and ongoing cost reduction efforts.
As of June 30, 2025, the company had $628 million in cash and cash equivalents compared with $747 million as of March 31, 2025. While the $175 million was recognized in the second quarter, Novavax expects its receipt in Q3.
Due to its reliance on Sanofi's sales forecasts for certain components, Novavax continues to withhold its full-year revenue guidance. However, the company has raised its adjusted revenue framework and now expects to generate between $1 billion and $1.05 billion, implying an increase from the prior projection of $0.98-$1.03 billion. This revision is largely due to the receipt of $20 million for cost reimbursement from Sanofi for a post-marketing commitment (PMC) requested by the FDA on Nuvaxovid later this year.
Because of the above receipt, Novavax also raised its full-year projection for combined R&D and SG&A expenses, which are now expected in the range of $495-$545 million (previously: $475-$525 million).
Novavax expects the transfers of marketing authorization to Sanofi for the U.S. and European Union (EU) markets in the fourth quarter of 2025. The completion of these events will trigger an additional $50 million in combined milestone payments.
In June, Novavax reported initial cohort data of a late-stage study evaluating its experimental COVID-influenza combination (CIC) and stand-alone influenza vaccine candidates. The results showed that the vaccines generated robust immune responses, which were comparable to Nuvaxovid and Sanofi’s influenza vaccine, Fluzone HD.
Novavax emphasized that the above study was not designed to demonstrate statistical significance but to provide preliminary immunogenicity data. It intends to use these findings to design another late-stage study, which could potentially support regulatory submissions, if successful. NVAX is also exploring strategic collaborations to finance further development and potential commercialization of both candidates.
The company remains on track to initiate the PMC study later this year and expects to incur $70-$90 million to complete the same. This study will be conducted by Novavax, with 30% of the costs to be reimbursed by Sanofi.
Last month, Novavax announced preclinical data on its H5N1 avian pandemic influenza vaccine candidate. Results showed that this vaccine generated robust immune responses by either single or two-dose intranasal or intramuscular administration in primates.
NVAX also announced that it has entered into ‘material transfer agreements’ with three unnamed pharmaceutical companies during the first quarter of 2025 to explore the utility of its patented Matrix-M in their portfolios.
Novavax currently carries a Zacks Rank #3 (Hold).

Novavax, Inc. price | Novavax, Inc. Quote
Some better-ranked stocks from the industry include CorMedix CRMD and Genmab GMAB, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Estimates for CorMedix’s 2025 EPS have increased from 93 cents to 97 cents over the past 60 days, while the same for 2026 has increased from $1.64 to $1.65. CRMD shares have surged 48% year to date.
CorMedix’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 25.82%.
Estimates for Genmab’s 2025 EPS have increased from $1.52 to $1.58 over the past 60 days, while the same for 2026 has increased from $1.85 to $1.93. GMAB shares have gained 3% year to date.
Genmab’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 14.90%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 3 hours | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 | |
| Feb-27 |
Novavax Stock Hits Over 1-Year High: Is Sanofis Flu-COVID Shot The Post-Pandemic Growth Driver?
NVAX -9.38% SNY
New feeds test provider finance
|
| Feb-27 | |
| Feb-27 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 | |
| Feb-26 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite