|
|
|

|
|||||

|
|
Travere Therapeutics TVTX announced that the FDA, following further review of the supplemental new drug application (sNDA) for Filspari (sparsentan) in focal segmental glomerulosclerosis ("FSGS"), has determined that an advisory committee meeting is no longer required. The sNDA remains under active review, with a final decision from the regulatory body expected on Jan. 13, 2026. TVTX’s shares rose 26.2% yesterday in response to the encouraging update.
Per Travere Therapeutics, FSGS is a rare kidney disorder affecting more than 40,000 patients in the United States and a comparable number in the European Union. The disease is marked by progressive kidney scarring and proteinuria (protein in the urine), where leaked protein damages kidney structures and drives disease progression. Patients often experience edema, low blood albumin, abnormal lipid levels and hypertension, with many ultimately progressing to kidney failure. Currently, there are no FDA-approved treatments for FSGS.
Subject to approval, Filspari would become the first treatment specifically indicated for FSGS. The drug is an oral, non-immunosuppressive potential therapy designed to address podocyte injury, a critical factor in the progression of FSGS.
Travere Therapeutics’ sNDA for Filspari is based on robust clinical evidence from two of the largest head-to-head interventional studies in FSGS, the phase III DUPLEX and phase II DUET studies. In both studies, treatment with the drug resulted in rapid, superior and sustained reductions in proteinuria compared with the maximum labeled dose of Sanofi’s Avapro (irbesartan) across adult and pediatric patients.
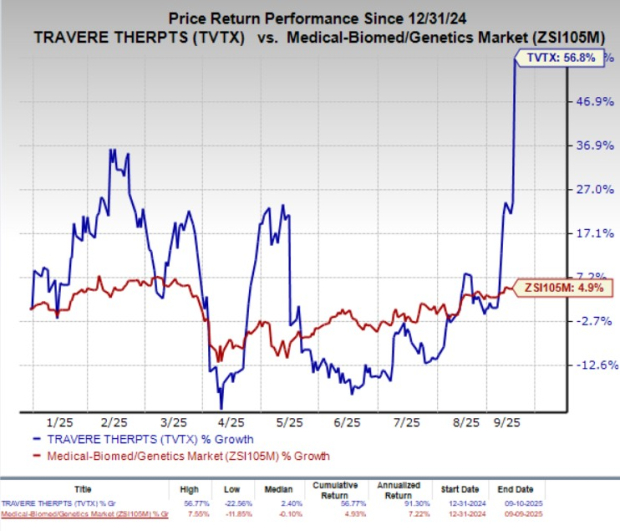
Year to date, TVTX stock has surged 56.8% compared with the industry’s 4.9% growth.
Results from the DUPLEX study demonstrated statistically significant and clinically meaningful proteinuria remission at 36 weeks upon treatment with Filspari, which was maintained for up to two years. Importantly, patients in the DUPLEX study who reached partial or complete remission had a 67% to 77% lower risk of kidney failure, with stronger benefits seen at more stringent remission thresholds. These findings are consistent with independent evidence from the PARASOL workgroup, underscoring the critical role of proteinuria reduction in FSGS outcomes.
The DUPLEX study, however, did not meet the primary efficacy eGFR slope endpoint more than 108 weeks of treatment with the drug. In the DUET study, Filspari achieved the primary efficacy endpoint in the combined treatment group, delivering more than double the reduction in proteinuria compared with Avapro. The drug also demonstrated a favorable safety and tolerability profile, with results consistent across studies and comparable to Avapro. This positions Filspari as a potentially meaningful advancement for patients with FSGS, who currently face a lack of approved treatment options.
Last year, the FDA granted full approval to Travere Therapeutics’ Filspari for the IgA nephropathy (IgAN) indication, a rare progressive kidney disease. Following this decision, Filspari is approved for slowing kidney function decline in adults with primary IgAN who are at risk of disease progression. The FDA’s decision made Filspari the only non-immunosuppressive medication in the IgAN space.
Filspari was initially granted accelerated approval in 2023 to reduce proteinuria in adults with primary IgAN who are at risk of rapid disease progression. The full approval expanded the drug’s total addressable patient population to treat those who are at high/low risk of disease progression.
Despite the full approval, the drug was added to the FDA’s Risk Evaluation and Mitigation Strategies (“REMS”) program. Doctors must monitor patients' liver enzymes before starting treatment, every month for the first year and then every three months during treatment. Filspari comes with a boxed warning for severe birth defects in case the drug is taken during pregnancy.
Last month, Travere Therapeutics gained a regulatory boost after the FDA approved a streamlined REMS for Filspari in IgAN, reducing the frequency of liver function monitoring from monthly to quarterly and eliminating the embryo-fetal toxicity monitoring requirement. Backed by robust clinical and real-world safety data, the update lowers treatment burden, simplifies access and could accelerate physician adoption. The move further strengthens Filspari’s position as the leading foundational therapy in IgAN, reinforcing its long-term commercial potential.

Travere Therapeutics, Inc. price-consensus-chart | Travere Therapeutics, Inc. Quote
Travere Therapeutics currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are CorMedix CRMD, Pharming Group PHAR and Kiniksa Pharmaceuticals KNSA, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for CorMedix’s earnings per share have increased from $1.10 to $1.52 for 2025. During the same time, earnings per share estimates for 2026 have increased from $1.46 to $2.12. Year to date, shares of CRMD have surged 57.8%.
CorMedix’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 34.85%.
In the past 60 days, estimates for Pharming Group’s 2025 loss per share have narrowed from 40 cents to 10 cents. For 2026, PHAR’s earnings per share estimate has improved from 7 cents to 27 cents. PHAR stock has risen 34.2% year to date.
Pharming Group’s earnings beat estimates in two of the trailing four quarters and missed on the remaining two occasions, delivering an average negative surprise of 39.14%.
In the past 60 days, estimates for Kiniksa Pharmaceuticals’ 2025 earnings per share have increased from 74 cents to $1.03. Earnings per share estimate for 2026 has increased from $1.19 to $1.60 during the same period. KNSA stock has surged 83.2% year to date.
Kiniksa Pharmaceuticals’ earnings beat estimates in two of the trailing four quarters and missed on the remaining two occasions, delivering an average negative surprise of 330.56%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-27 | |
| Feb-26 | |
| Feb-26 | |
| Feb-25 | |
| Feb-25 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-23 | |
| Feb-23 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite