|
|
|

|
|||||

|
|
Shares of uniQure N.V. QURE skyrocketed 247.7% on Wednesday after the company reported meeting key goals in the pivotal early to mid-stage study of its investigational gene therapy, AMT-130, for the treatment of Huntington’s disease.
In accordance with the prospectively defined statistical analysis plan, which was aligned with and submitted to the FDA for the pivotal phase I/II study, uniQure evaluated clinical outcomes in 29 patients treated with AMT-130 (17 receiving the high dose, 12 the low dose). At the 36-month follow-up, 12 patients from each dose group were assessed. Outcomes for both dose groups were compared against a propensity score-matched external control derived from the Enroll-HD natural history dataset.
Per uniQure N.V.’s data readout, the pivotal phase I/II Huntington’s disease study met its prespecified primary endpoint, with the high dose of AMT-130 achieving a statistically significant 75% slowing of disease progression as measured by the composite Unified Huntington’s Disease Rating Scale (cUHDRS) at 36 months compared to a propensity score-matched external control. Treated patients experienced a mean change in cUHDRS from baseline of -0.38 compared with a -1.52 change observed in the external control group.
Please note that the cUHDRS has been demonstrated to be the most sensitive measurement of clinical progression in Huntington’s disease patients.
The study also met its key secondary endpoint by achieving a statistically significant 60% slowing of disease progression as measured by Total Functional Capacity (TFC) at 36 months compared to a propensity score-matched external control. Patients treated with the high dose of AMT-130 showed a mean change in TFC from baseline of -0.36 compared with -0.88 in the propensity score-matched external control group.
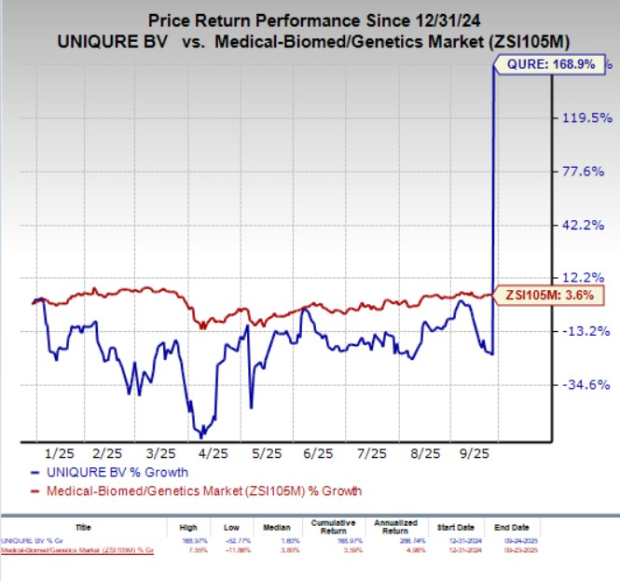
Year to date, shares of uniQure N.V. have surged 168.9% compared with the industry’s 3.6% growth.
Data from the pivotal phase I/II study also indicated a favorable dose-dependent response to AMT-130 across multiple other secondary endpoints assessing motor and cognitive function. Patients receiving the high dose of AMT-130 showed an 88% slowing of disease progression on the Symbol Digit Modalities Test, a 113% slowing on the Stroop Word Reading Test and a 59% slowing on the Total Motor Score, compared with propensity score-matched external control.
Additionally, cerebrospinal neurofilament light protein, an important biomarker linked to neurodegeneration and disease severity in Huntington’s disease, decreased by an average of 8.2% from baseline.
Supportive analyses using alternative external controls, including TRACK-HD and PREDICT-HD datasets, were aligned with the primary findings, reinforcing the potential of AMT-130 to slow disease progression. QURE further reported that the candidate was well-tolerated in the pivotal phase I/II study with a manageable safety profile at both doses. Adverse events related to treatment were mostly mild in severity.
Based on the encouraging data readout, uniQure N.V. plans to engage with the FDA at a meeting expected later this year, aiming to submit a biologics license application for AMT-130 to treat Huntington's disease in the first quarter of 2026.
Huntington's disease is a genetic disorder that causes the progressive breakdown of nerve cells in the brain, which leads to a decline in cognitive and physical abilities, often resulting in movement, thinking and psychiatric problems. uniQure N.V. enjoys the FDA’s Regenerative Medicine Advanced Therapy and Breakthrough Therapy designations for AMT-130 to treat Huntington’s disease.
Apart from AMT-130, uniQure N.V.’s wholly-owned clinical pipeline comprises several other candidates that are currently undergoing early to mid-stage development for the treatment of patients with refractory mesial temporal lobe epilepsy, amyotrophic lateral sclerosis and Fabry disease.
Please note that the company also markets an internally developed gene therapy for the treatment of hemophilia B in the United States and the EU under the brand name Hemgenix. The approvals in the United States and EU markets in 2022 and 2023, respectively, marked a significant milestone in the field of genomic medicine, bringing a new treatment approach for patients living with hemophilia.

uniQure N.V. price-consensus-chart | uniQure N.V. Quote
uniQure N.V. currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are CorMedix CRMD, Pharming Group PHAR and Kiniksa Pharmaceuticals KNSA, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for CorMedix’s earnings per share have increased from 97 cents to $1.24 for 2025. During the same time, earnings per share estimates for 2026 have increased from $1.65 to $2.09. Year to date, shares of CRMD have surged 38.7%.
CorMedix’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 34.85%.
In the past 60 days, estimates for Pharming Group’s 2025 loss per share have narrowed from 40 cents to 10 cents. For 2026, PHAR’s earnings per share estimate has improved from 7 cents to 27 cents. PHAR stock has risen 46.3% year to date.
Pharming Group’s earnings beat estimates in two of the trailing four quarters and missed on the remaining two occasions, delivering an average negative surprise of 39.14%.
In the past 60 days, estimates for Kiniksa Pharmaceuticals’ 2025 earnings per share have increased from 74 cents to $1.03. Earnings per share estimate for 2026 has increased from $1.19 to $1.60 during the same period. KNSA stock has surged 82.4% year to date.
Kiniksa Pharmaceuticals’ earnings beat estimates in two of the trailing four quarters and missed on the remaining two occasions, delivering an average negative surprise of 330.56%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 3 hours | |
| 7 hours | |
| 7 hours | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-18 | |
| Feb-17 | |
| Feb-17 | |
| Feb-14 | |
| Feb-13 | |
| Feb-12 | |
| Feb-12 | |
| Feb-11 | |
| Feb-11 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite