|
|
|

|
|||||

|
|
Pacira BioSciences PCRX announced that it has dosed the first patient in a mid-stage study of pipeline candidate PCRX-201 (enekinragene inzadenovec) for the treatment of osteoarthritis (OA) of the knee.
PCRX-201 is an advanced gene therapy designed to use the company’s proprietary HCAd vector platform. It is administered by injecting directly into the knee joint. The candidate’s unique mechanism of action reduces chronic inflammation and pain while improving function. A key feature of Pacira’s PCRX-201 is its inducible promoter, mimicking the body's natural response by activating IL-1Ra expression during inflammation and deactivating it once inflammation subsides.
Per management, currently available therapies for this indication rely on outdated mechanisms of action that only provide up to three to six months of relief. In stark contrast, PCRX-201 has previously demonstrated unprecedented pain relief and durability across all levels of OA severity for at least two years.
Pacira believes PCRX-201 can target the chronic inflammatory processes that drive OA joint degeneration over time, with localized administration that remains confined to the joint.
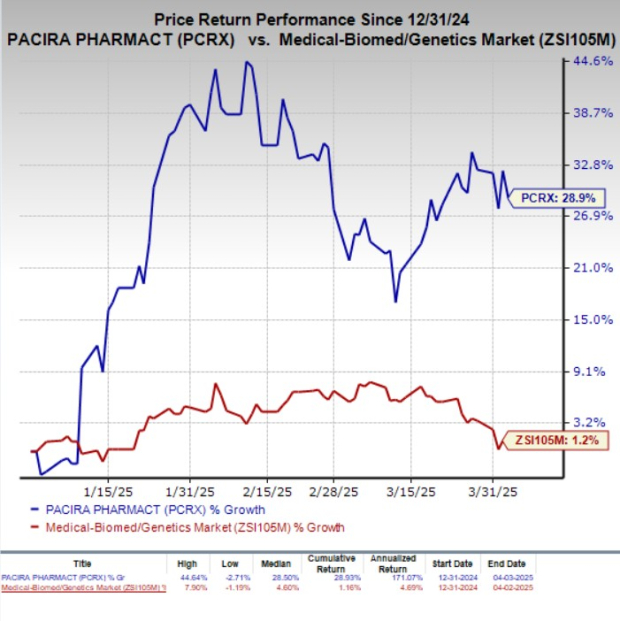
Year to date, shares of PCRX have rallied 28.9% compared with the industry’s 1.2% growth.
The phase II ASCEND study of PCRX-201 for OA knee is expected to enroll approximately 135 patients and will be conducted in two parts — A and B.
The enrolled patient population will be randomized equally into three cohorts to receive either of the two dosage strengths of PCRX-201 being evaluated or saline. Per Pacira, all cohorts in the ASCEND study will undergo pretreatment with an intra-articular corticosteroid (methylprednisolone 40 mg), a standard gene therapy approach to enhance tolerability and gene transfer efficiency.
Part A of the study will enroll about 45 patients, while Part B will include approximately 90 patients. PCRX anticipates releasing top-line results from Part A by the end of 2026.
The primary goal of both Parts A and B is to assess the safety of PCRX-201 compared to saline, both with steroid pretreatment, by tracking adverse events from week 1 to week 52. Secondary and exploratory endpoints focus on efficacy, measuring pain and physical function changes at Weeks 38 and 52 using various metrics.
The study will also evaluate biomarkers, including structural endpoints, immunogenicity and biodistribution. All patients will be monitored for five years.

Pacira BioSciences, Inc. price-consensus-chart | Pacira BioSciences, Inc. Quote
Pacira’s commercial portfolio comprises three marketed drugs — Exparel, Zilretta and iovera.
Exparel is PCRX’s flagship pain-management product, which was initially launched in 2012. The drug is indicated for postsurgical local analgesia in patients aged six years and older. It is also indicated for regional analgesia in adults via an interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa and femoral nerve block in the adductor canal.
Zilretta, on the other hand, is approved as an extended-release intra-articular therapy providing relief to OA patients with knee pain. The iovera system is a hand-held medical device used to deliver a precise, controlled application of cold temperature to targeted nerves.
In 2024, Pacira generated net product revenues worth $697.2 million, representing growth of 4% on a year-over-year basis.
Pacira currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the sector are Bayer BAYRY, Dynavax Technologies Corporation DVAX and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
In the past 30 days, estimates for Bayer’s earnings per share have increased from $1.14 to $1.19 for 2025. During the same time, earnings per share have increased from $1.23 to $1.28 for 2026. Year to date, shares of Bayer have gained 20.3%.
BAYRY’s earnings matched estimates in two of the trailing three quarters while missing the same on the remaining occasion, the average negative surprise being 19.61%.
In the past 30 days, estimates for Dynavax’s earnings per share have remained constant at 33 cents for 2025. During the same time, earnings per share have remained constant at 57 cents for 2026. Year to date, shares of DVAX have gained 0.6%.
DVAX’s earnings beat estimates in three of the trailing four quarters while missing the same on the remaining occasion, the average surprise being 9.58%.
In the past 30 days, the estimate for Corcept Therapeutics’ 2025 earnings per share has increased from $1.84 to $1.87. The estimate for 2026 earnings per share has increased from $3.05 to $3.16. Year to date, shares of Corcept Therapeutics have gained 57.5%.
CORT’s earnings beat estimates in three of the trailing four quarters and missed once, delivering an average surprise of 20.08%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-17 | |
| Feb-16 | |
| Feb-12 | |
| Feb-12 | |
| Feb-09 | |
| Feb-04 | |
| Feb-04 | |
| Feb-03 | |
| Jan-30 | |
| Jan-29 | |
| Jan-28 | |
| Jan-27 | |
| Jan-27 | |
| Jan-25 | |
| Jan-23 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite