|
|
|

|
|||||

|
|
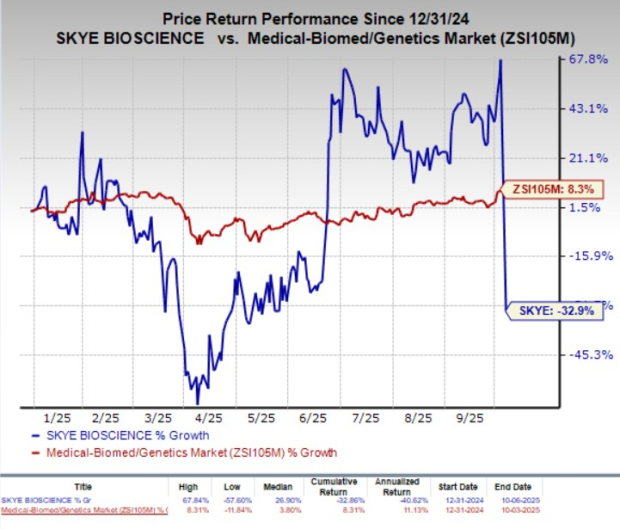
Shares of Skye Bioscience SKYE crashed 60% on Monday after the company failed to meet the key goal in a mid-stage proof-of-concept study of its only pipeline candidate, nimacimab, for obesity.
The phase IIa CBeyond study is evaluating the efficacy, safety and pharmacokinetics of nimacimab, a peripherally-restricted CB1 inhibitor antibody, in 136 adults with overweight or obesity, including individuals with a BMI ≥27 kg/m² with at least one comorbidity.
Patients were randomized into four groups, nimacimab 200 mg, placebo, nimacimab in combination with Novo Nordisk’s NVO blockbuster obesity drug, Wegovy (semaglutide), or placebo in combination with Wegovy, and treated weekly for 26 weeks. The latest data readout is from this 26-week treatment period.
Per the data readout from the phase IIa CBeyond obesity study, the nimacimab 200 mg monotherapy arm did not achieve the primary weight loss goal, showing a body weight reduction of 1.52% compared to 0.26% with placebo at week 26.
Skye Bioscience reported that preliminary pharmacokinetic analysis indicated that nimacimab exposure was lower than expected and correlated with response, suggesting that the 200 mg weekly subcutaneous dose was suboptimal and that higher doses should be evaluated.
Year to date, SKYE stock has plunged 32.9% against the industry’s 8.3% growth.
However, in the combination group, treatment with the combo therapy of nimacimab 200 mg and Novo Nordisk’s Wegovy led to a clinically meaningful weight loss of 13.2% compared to treatment with Wegovy alone, resulting in a weight loss of 10.25%. Additionally, no plateau was observed through the 26-week treatment period in the combination arm, supporting further investigation of nimacimab in combination with incretin-based therapies.
Skye Bioscience also reported that 100% of the enrolled patients in the combination arm achieved more than 5% weight loss compared to 85% receiving only NVO’s Wegovy, and 67% achieved more than 10% weight loss in the combo arm compared to 50% with Wegovy alone.
At the tested dose and exposure levels, nimacimab 200 mg showed a favorable safety profile and tolerability comparable to placebo. When combined with Wegovy, there was no increase in gastrointestinal adverse events and no treatment-related neuropsychiatric adverse events were reported.
Skye Bioscience continues to evaluate the data from the 26-week treatment period of the CBeyond obesity study and is expected to report detailed data at an upcoming medical conference.
Skye Bioscience is continuing the phase IIa CBeyond obesity study of nimacimab beyond the initial 26-week treatment period for another 26-week extension period for a potential full treatment duration of 52 weeks with a 13-week follow-up period.
In the combination groups, patients will continue blinded treatment with either nimacimab or placebo, along with Novo Nordisk’s Wegovy. Those in the monotherapy group will receive nimacimab 300 mg during the extension phase. Enrollment for the extension is complete, and Skye Bioscience plans to report the study results in the first quarter of 2026.
Skye Bioscience believes several factors justify testing higher doses of nimacimab, including favorable preclinical toxicology and modeling data, evidence of dose-dependent weight loss in both monotherapy and GLP-1 combination studies and the strong safety profile observed in the CBeyond obesity study. The company is continuing to assess the data to define next steps, which may include a follow-up phase II study in addition to the ongoing phase IIa extension study.
Novo Nordisk is currently the market leader in GLP-1 therapies for obesity. Wegovy is a major revenue driver for NVO, recording sales of $5.41 billion (DKK 36.9 billion) during the first half of 2025, up 78% year over year on strong prescription growth.
The drug is also indicated for reducing major cardiovascular events, easing HFpEF symptoms, and relieving osteoarthritis-related knee pain in obesity. The FDA recently granted accelerated approval to Wegovy as the first GLP-1 therapy to treat noncirrhotic metabolic dysfunction-associated steatohepatitis with moderate-to-advanced liver fibrosis. An oral formulation of the drug is currently being reviewed by the FDA for obesity, with a decision expected by year-end.

Skye Bioscience, Inc. price-consensus-chart | Skye Bioscience, Inc. Quote
Skye Bioscience currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the drug sector are Amicus Therapeutics FOLD and Avadel Pharmaceuticals AVDL, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Amicus Therapeutics’ 2025 earnings per share have remained constant at 31 cents. Earnings per share estimates for 2026 have increased from 68 cents to 69 cents during the same period. FOLD stock has lost 13.3% year to date.
Amicus Therapeutics’ earnings beat estimates in one of the trailing four quarters while missing the same on the remaining three occasions, the average negative surprise being 24.38%.
In the past 60 days, estimates for Avadel Pharmaceuticals’ 2025 earnings per share have moved up from 19 cents to 25 cents. Earnings per share estimates for 2026 have increased from 77 cents to 85 cents during the same period. AVDL stock has surged 38.2% year to date.
Avadel Pharmaceuticals’ earnings beat estimates in three of the trailing four quarters, while meeting the same on the remaining occasion, the average surprise being 119.64%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 40 min | |
| 2 hours | |
| 3 hours | |
| 3 hours | |
| 4 hours | |
| 5 hours | |
| 5 hours | |
| 6 hours |
Novo Nordisk Licenses Vivtex Tech for Up to $2.1 Billion to Deepen Obesity Push
NVO
The Wall Street Journal
|
| 7 hours | |
| 7 hours |
Novo Nordisk, Vivtex to Develop Obesity Drugs in Deal Valued at Up to $2.1 Billion
NVO
The Wall Street Journal
|
| 7 hours | |
| 8 hours | |
| 9 hours | |
| 12 hours | |
| 13 hours |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite