|
|
|

|
|||||

|
|
Shares of ImmunityBio IBRX surged 17.4% after it announced a recent face-to-face meeting with senior FDA officials to align on the regulatory path forward for Anktiva in an additional indication. IBRX is planning a resubmission of its supplemental biologics license application (sBLA) seeking the approval of Anktiva (nogapendekin alfa inbakicept) plus Bacillus Calmette-Guérin (BCG) for BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with papillary tumors. The sBLA was originally submitted in 2025 and received a refusal to file letter from the FDA.
Please note that Anktiva is already approved in the United States, the EU and the United Kingdom in combination with BCG to treat adults with BCG-unresponsive NMIBC with carcinoma in situ (CIS), with or without papillary tumors.
At the latest FDA meeting, ImmunityBio presented a comprehensive update on the clinical progress of its papillary disease program, highlighting more than five years of follow-up data supporting the papillary indication. The combination regimen also demonstrated a safety profile consistent with the currently approved CIS indication, with or without papillary tumors. In addition, several leading urologists shared real-world treatment experiences for patients with BCG-unresponsive disease, where radical cystectomy is often the only remaining option.
Following these discussions, the FDA requested additional information to support a potential resubmission of ImmunityBio’s Anktiva plus BCG sBLA for the papillary indication. IBRX has completed the requested analyses and plans to submit the package within a month.
The company further clarified that the additional information requested does not contemplate the initiation or design of a new clinical study. The meeting also addressed current standards of care, limitations of chemotherapy, patient management challenges, and interpretation of the company’s clinical data.
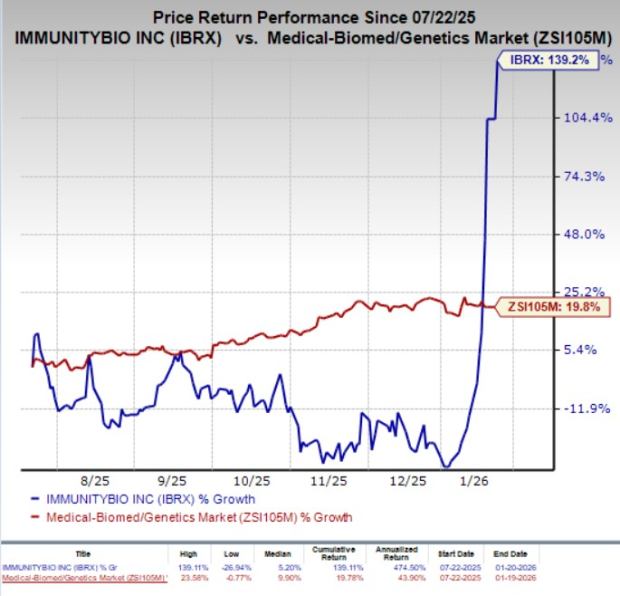
In the past six months, ImmunityBio shares have skyrocketed 139.2% compared with the industry’s 19.8% growth.
ImmunityBio’s proposed sBLA resubmission for Anktiva in BCG-unresponsive papillary NMIBC is supported by long-term data from the phase II/III QUILT-3.032 study (Cohort B), which enrolled 80 patients with high-grade papillary-only disease. The study met its primary endpoint, delivering a 12-month disease-free survival rate of 58.2%, demonstrating meaningful and durable disease control in a high-risk population with limited treatment options.
Longer-term follow-up further highlights the durability of the response. Disease-specific survival reached 96% at 36 months, with the median not yet reached, while progression-free survival remained strong at 94.9% at 12 months and 83.1% at 36 months. These outcomes indicate sustained control of disease and a durable reduction in the risk of progression to muscle-invasive bladder cancer. Importantly, Anktiva also demonstrated a compelling bladder-sparing benefit. Cystectomy-free survival was 92.2% at 12 months and 81.8% at 36 months, meaning more than 80% of patients avoided radical surgery through three years of follow-up.
High-grade papillary NMIBC that no longer responds to BCG represents a major unmet need, as no targeted therapies are currently approved for these patients. Standard of care for this indication has been radical cystectomy (complete removal of the bladder), an invasive surgery associated with significant morbidity and impact on quality of life.
ImmunityBio is pursuing this indication to offer a bladder-sparing alternative, and long-term data from the QUILT-3.032 study suggest that Anktiva plus BCG can deliver durable remissions while delaying or avoiding surgery for most responders. If approved, Anktiva would become the first immunotherapy for BCG-unresponsive papillary NMIBC, building on its CIS approval and potentially reshaping the treatment landscape for these patients.
ImmunityBio is also in discussions with global regulators, including the European Medicines Agency and other regional authorities, to pursue a label expansion of the combo into papillary-only disease following U.S. approval.

ImmunityBio, Inc. price-consensus-chart | ImmunityBio, Inc. Quote
ImmunityBio currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Amicus Therapeutics FOLD, Alkermes ALKS and Krystal Biotech KRYS, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 60 days, estimates for Amicus Therapeutics’ 2026 EPS have decreased from 67 cents to 65 cents. Shares of FOLD have surged 130.1% over the past six months.
Amicus Therapeutics’ earnings beat estimates in one of the trailing four quarters, missing the mark on the other three occasions, delivering an average negative surprise of 20.21%.
Over the past 60 days, 2026 EPS estimates for Alkermes have increased from $1.54 to $1.90. Shares of ALKS have gained 17.5% over the past six months.
Alkermes’ earnings beat estimates in three of the trailing four quarters, missing on the remaining occasion, with the average surprise being 4.58%.
Over the past 60 days, estimates for Krystal Biotech’s EPS for 2026 have risen to $8.49 from $8.34. KRYS stock has rallied 80.9% over the past six months.
Krystal Biotech’s earnings beat estimates in three of the trailing four quarters and missed in the remaining quarter, with the average surprise being 40.43%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 55 min | |
| 4 hours | |
| 5 hours | |
| 6 hours | |
| 7 hours | |
| 7 hours | |
| 10 hours | |
| Feb-21 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite