|
|
|

|
|||||

|
|
Johnson & Johnson JNJ delivered strong fourth-quarter 2025 results, with both the top and bottom lines exceeding expectations. Total revenues rose 9.1% to $24.56 billion, while adjusted EPS of $2.46 rose 20.6% year over year.
Despite the loss of exclusivity (“LOE”) of its multi-billion-dollar product, Stelara, sales in J&J’s Innovative Medicines unit rose 10.0% year over year to $15.76 billion in the fourth quarter. Its MedTech segment also showed continued improvement, driven by strong performance in Cardiovascular, Surgery and Vision. Sales in the MedTech segment rose 7.5% to $8.8 billion in the fourth quarter.
J&J’s 2026 guidance was also above expectations. J&J said it expects sales in the range of $100.0 billion-$101.0 billion in 2026. Adjusted earnings per share are expected to be in the range of $11.43-$11.63. J&J expects accelerated growth in both the Innovative Medicine and MedTech segments in 2026.
However, a single quarter’s results are not so important for long-term investors, and the focus should rather be on the company’s strong fundamentals. Let’s understand the company’s strengths and weaknesses to better analyze how to play J&J stock in the post-earnings scenario.
J&J’s Innovative Medicine unit is showing a growth trend. The segment’s sales rose 4.1% on an organic basis in 2025 despite Stelara LOE and the negative impact of the Part D redesign. Growth was driven by J&J’s key drugs like Darzalex, Erleada and Tremfya. New drugs like Carvykti, Tecvayli, Talvey, Rybrevant and Spravato also contributed significantly to growth.
The segment recorded three consecutive quarters of sales of more than $15 billion despite the LOE of Stelara. The segment, for the first time, generated more than $60 billion in full-year sales in 2025, with13 brands growing in double digits.
In 2026, J&J expects accelerated growth in the Innovative Medicine segment despite the Stelara LOE impact. The growth is expected to be driven by its key products, such as Darzalex, Tremfya, Spravato, Carvykti and Erleada, as well as new launches like Rybrevant plus Lazcluze in non-small cell lung cancer and Caplyta in major depressive disorder (MDD). J&J expects a more pronounced impact from new products in 2026 than in 2025. It expects the Innovative Medicine business to grow 5% to 7% from 2025 to 2030.
In 2025, J&J invested over $32 billion in R&D and M&A, including the acquisitions of Intra-Cellular Therapies and Halda Therapeutics.
The company rapidly advanced its pipeline in the year, attaining significant clinical and regulatory milestones that will help drive growth through the back half of the decade. In 2025, it gained approval for new products like Inlexzoh/TAR-200, a first-of-its-kind drug-releasing system, for treating high-risk non-muscle invasive bladder cancer and Imaavy (nipocalimab) for treating generalized myasthenia gravis. J&J believes that nipocalimab has a pipeline-in-a-product potential. Regulatory applications were recently filed for another key candidate, Icotyde/icotrokinra, for moderate-to-severe plaque psoriasis. J&J believes that Icotyde/icotrokinra has the potential to revolutionize the treatment of plaque psoriasis with a once-a-day pill.
Three of J&J’s new cancer drugs are Carvykti, a BCMA CAR-T therapy for relapsed or refractory multiple myeloma; Tecvayli, for relapsed or refractory multiple myeloma; and Talvey, a novel bispecific therapy for heavily pretreated multiple myeloma. These drugs have also begun to contribute to top-line growth. Combined, they generated $3.0 billion in sales in 2025.
J&J’s acquisition of Intra-Cellular Therapies added antidepressant drug, Caplyta, to its neuroscience portfolio, which is approved for the treatment of schizophrenia, depression in both bipolar 1 and 2, and MDD.
J&J believes 10 of its new products/pipeline candidates in the Innovative Medicine segment have the potential to deliver peak sales of $5 billion, including Talvey, Tecvayli, Imaavy, Caplyta, Inlexzo, Rybrevant, plus Lazcluze and Icotyde.
J&J’s MedTech business has improved in the past three quarters, driven by the acquired cardiovascular businesses, Abiomed and Shockwave, as well as Surgical Vision and wound closure in Surgery. Improvements in J&J’s electrophysiology business also drove the growth. MedTech sales rose 4.3% on an organic basis in 2025.
Moreover, the potential separation of its Orthopaedics franchise into a standalone orthopedics-focused company, called DePuy Synthes, should improve its MedTech unit’s growth and margins. The Orthopaedics franchise has been a slow-growth business for J&J.
In 2026, J&J expects better growth in the MedTech business than 2025 levels, driven by increased adoption of newly launched products across Cardiovascular, Surgery and Vision portfolios. Some key new product launches are VARIPULSE in Electrophysiology, ETHICON4000 in Surgery and the OASYS MAX family in Vision.
However, the company continues to face headwinds in China. Sales in China are being hurt by the impact of the volume-based procurement (VBP) program, which is a government-driven cost containment effort in China. J&J expects continued impacts from VBP issues in China in 2026.
J&J lost U.S. patent exclusivity of Stelara in 2025. Stelara was a key top-line driver for J&J, accounting for around 18% of J&J’s Innovative Medicine unit’s sales in 2024, before it lost patent exclusivity in 2025.
Several biosimilar versions of J&J’s multi-billion-dollar immunology drug, Stelara, were launched in the United States in 2025 as the drug lost patent exclusivity.
According to patent settlements and license agreements, Amgen AMGN, Teva Pharmaceutical Industries TEVA, Samsung Bioepis/Sandoz and some other companies launched Stelara biosimilars in 2025. Stelara’s LOE negatively impacted the Innovative Medicines segment’s growth by 1040 basis points in 2025. In 2026, J&J expects Stelara LOE impact to be more pronounced. In addition, J&J expects generic impact for both Simponi and Opsumit to begin in 2026 as the drugs lose patent protection.
In addition, sales are being hurt by the impact of the Medicare Part D redesign under the Inflation Reduction Act (IRA). The Part D redesign is mainly affecting sales of drugs like Stelara, Erleada and pulmonary hypertension drugs.
J&J faces more than 73,000 lawsuits for its talc-based products, primarily baby powders. The lawsuits allege that its talc products contain asbestos, which caused many women to develop ovarian cancer. J&J insists that its talc-based products are safe and do not cause cancer. The company permanently discontinued the sales of the talc-based Johnson’s Baby Powder.
In April 2025, a bankruptcy court in Texas rejected J&J’s proposed bankruptcy plan to settle its talc lawsuits after a two-week trial in Houston. J&J has gone back to the traditional tort system to fight the lawsuits individually, with its bankruptcy strategy to settle the lawsuits failing for the third time.
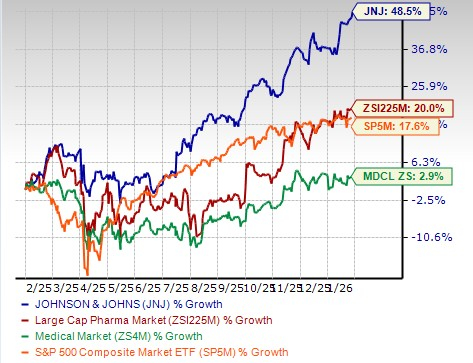
J&J’s shares have outperformed the industry in the past year. The stock has risen 48.5% in the past year compared with a 20.0% increase of the industry. The stock has also outperformed the sector and the S&P 500 Index, as seen in the chart below.
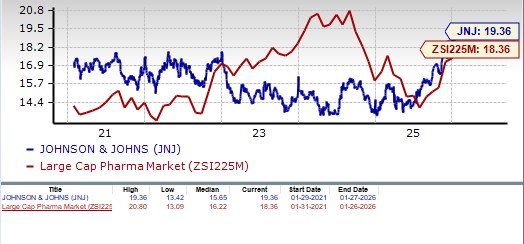
From a valuation standpoint, J&J is slightly expensive. Going by the price/earnings ratio, the company’s shares currently trade at 19.36 forward earnings, higher than 18.36 for the industry. The stock is also trading above its five-year mean of 15.65.
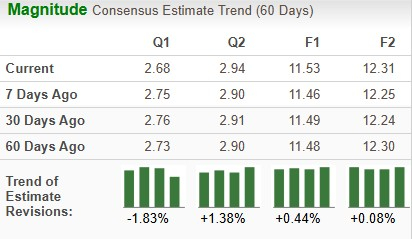
The Zacks Consensus Estimate for 2026 earnings has risen from $11.46 to $11.53 over the past seven days, while that for 2027 has gone up from $12.25 per share to $12.31 per share over the same timeframe. The EPS estimates have moved up in response to J&J’s optimistic 2026 financial outlook.
J&J’s biggest strength is its diversified business model, as it operates through pharmaceuticals and medical devices divisions. It has more than 275 subsidiaries and boasts 28 platforms or products with more than $1 billion in annual sales. Its diversification helps it to withstand economic cycles more effectively. It also boasts strong cash flows and has consistently increased its dividends for 63 consecutive years.
J&J outperformed financial expectations in 2025 and looks optimistic for continued strong momentum in 2026 with a target to generate around $100 billion in revenues in the year. J&J expects sales growth in both segments to be higher in 2026.
Despite headwinds like the legal battle surrounding its talc lawsuits, the Stelara patent cliff, the upcoming LOE of key drugs Opsumit and Simponi and softness in MedTech China, J&J looks quite confident that it will be able to navigate these challenges.
J&J’s price appreciation, rising estimates, consistent earnings and sales growth, important new launches and significant pipeline progress suggest one should stay invested in this Zacks Rank #3 (Hold) stock for now. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite