|
|
|

|
|||||

|
|
Swiss pharma giant Roche Holding AG's RHHBY 2025 results were affected by currency headwinds.
Sales totaled $74.4 billion, which missed the Zacks Consensus Estimate of $81.4 billion. Earnings per American Depositary Receipt of $2.94 also missed the Zacks Consensus Estimate of $3.06.
The appreciation of the Swiss franc against most currencies, notably the US dollar, had an adverse impact on the results reported in Swiss francs compared to constant exchange rates (CER).
Sales grew 7% year over year at CER to CHF 61.5 billion, driven by strong demand for both drugs and diagnostics.
The company reports under two divisions — Pharmaceuticals and Diagnostics. All growth rates mentioned below are on a year-over-year basis and at CER.
Sales in the Pharmaceuticals Division grew 9% to CHF 47.7 billion, driven by strong growth in demand for its key drugs — Phesgo (breast cancer), Xolair (food allergies), Ocrevus (multiple sclerosis or MS), Hemlibra (hemophilia A) and Vabysmo (severe eye diseases).
The Diagnostics division’s sales totaled CHF 13.8 billion, up 2% as demand for pathology and molecular solutions continued to more than offset the impact of healthcare pricing reforms in China.
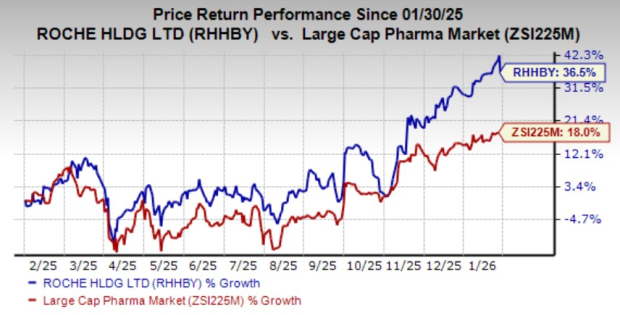
Roche’s shares have surged 36.5% year to date compared with the industry’s growth of 18%.
The top five growth drivers — Phesgo, Xolair, Hemlibra, Vabysmo and Ocrevus — generated total sales of CHF 21.4 billion, reflecting an increase of CHF 3.2 billion at CER compared to 2024.
The increase in the sales of these drugs offset the decline in sales of Avastin (various types of cancer), Herceptin (breast and gastric cancer), MabThera/Rituxan (blood cancer, rheumatoid arthritis), Esbriet (lung disease), Lucentis (severe eye diseases) and Actemra/RoActemra (rheumatoid arthritis) due to loss of exclusivity.
MS Ocrevus generated sales of CHF 7 billion, up 9%. Growth is boosted by strong demand of subcutaneous formulation.
Sales of hemophilia A drug Hemlibra surged 11% year over year to CHF 4.7 billion on continued global growth.
Vabysmo sales grew 12% to CHF 4.1 billion on strong demand in all regions. While the branded market in the United States contracted in 2025, a recovery is expected in 2026.
Immuno-oncology drug Tecentriq’s (for advanced lung cancer, urothelial cancer and breast cancer) sales were up 3% to CHF 3.6 billion.
Xolair sales surged 32% to CHF 3.1 billion. Sales were fueled by strong uptake in food allergy. However, a biosimilar launch is expected in the second half of 2026.
Perjeta’s sales were down 13% year over year to CHF 3 billion.
Actemra/RoActemra’s (rheumatoid arthritis and COVID-19) sales declined 2% year over year to CHF 2.5 billion.
Breast cancer drug Phesgo’s (a fixed-dose combination of Perjeta and Herceptin for subcutaneous injection) sales skyrocketed 48% year over year to CHF 2.4 billion due to strong conversion rates.
Breast cancer drug Kadcyla generated sales of CHF 2 billion, up 7% in 2025 driven by demand in adjuvant breast cancer.
Evrysdi generated sales of CHF 1.7 billion, up 13% year over year.
Sales of the lung cancer drug Alecensa rose 6% to CHF 1.6 billion, driven by growth in the United States and Japan.
Sales of Polivy surged 38% to CHF 1.5 billion, driven by strong uptake in first line diffuse large B-Cell lymphoma.
Sales of Rituxan/MabThera declined 4% to CHF 1.2 billion due to biosimilar erosion.
Activase/TNKase sales totaled CHF 1.1 billion, down 2%.
Herceptin sales plunged 22% on a year-over-year basis to CHF 1 billion due to biosimilar competition in various countries.
Blood cancer drug Gazyva/Gazyvaro’s sales totaled CHF 986 million, up 14% year over year.
Sales of Avastin, approved for multiple oncology indications, were down 17% to CHF 973 million due to biosimilar competition.
Pulmozyme (cystic fibrosis) sales rose 12% year over year to CHF 479 million.
Roche expects total sales to grow in the mid-single-digit range (at CER) in 2026. Core earnings per share are expected to grow in the high single-digit range. Roche expects to increase its dividend in Swiss francs further.
The European Commission approved Gazyva/Gazyvaro for adults with active lupus nephritis.
RHHBY also obtained approval for the subcutaneous form of Lunsumio for a type of blood cancer in the United States and EU.
The company also announced positive results on breast cancer candidate giredestrant from the phase III lidERA study. At the pre-specified interim analysis, adjuvant giredestrant significantly reduced the risk of invasive disease recurrence or death by 30% compared with standard-of-care endocrine therapy.
Roche also reported positive results from a late-stage study on its multiple sclerosis candidate fenebrutinib. The first (FENhance 2) of two pivotal relapsing multiple sclerosis studies met its primary endpoint, showing investigational fenebrutinib significantly reduced relapses compared to teriflunomide.
Fenebrutinib, an investigational Bruton’s tyrosine kinase (BTK) inhibitor, significantly reduced the annualized relapse rate compared to teriflunomide over a period of at least 96 weeks of treatment.
Roche’s performance in 2025 was weighed down by unfavorable foreign-exchange movements, as weakness in the U.S. dollar adversely impacted international sales.
Nonetheless, the company’s underlying operational performance remained solid. Strong growth from key products helped offset declining revenues from legacy drugs.
MS drug Ocrevus and ophthalmology drug Vabysmo continued their stellar performances. Growth in hemophilia treatment Hemlibra and breast cancer drug Phesgo also boosted RHHBY’s top line.
Roche has a strong and diversified pipeline spanning multiple therapeutic modalities. Positive data on its breast cancer candidate giredestrant and its multiple sclerosis candidate fenebrutinib have increased the likelihood of regulatory approval, which could serve as a meaningful catalyst for the stock.
Earlier this week, Roche announced positive top-line results from a phase II study of CT-388, an investigational dual GLP-1/GIP receptor agonist being developed for the treatment of obesity.
Results showed that a once-weekly subcutaneous injection of CT-388 achieved a statistically significant placebo-adjusted weight loss of 22.5% at 48 weeks at the highest dose tested (24 mg), without reaching a weight loss plateau.
Although Roche is a late entrant into the highly competitive obesity market — currently dominated by Eli Lilly LLY and Novo Nordisk NVO — the strength of CT-388’s data represents a significant positive development. Phase III obesity trials (Enith1 and Enith2) are expected to commence this quarter.
Eli Lilly currently leads the obesity market with its tirzepatide-based dual GLP-1/GIP receptor agonists, Mounjaro and Zepbound.
Arch rival Novo Nordisk commands a strong position with its semaglutide-based GLP-1 therapies, Ozempic and Wegovy, used in type 2 diabetes and obesity.
Both LLY and NVO generate a major chunk of their total revenues from their respective cardiometabolic drugs.
Roche currently carries a Zacks Rank #3 (Hold). A better-ranked large pharma company is Bayer BAYRY, which currently has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Shares of Bayer have skyrocketed 135.5% in the past year. Estimates for BAYRY’s 2026 earnings per share have increased from $1.38 to $1.51 over the past 60 days.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 3 hours | |
| 3 hours | |
| 5 hours | |
| 5 hours | |
| 5 hours | |
| 7 hours | |
| 7 hours | |
| 7 hours | |
| 7 hours | |
| 8 hours | |
| 8 hours | |
| 8 hours | |
| 9 hours |
Novo Nordisk Licenses Vivtex Tech for Up to $2.1 Billion to Deepen Obesity Push
NVO
The Wall Street Journal
|
| 9 hours | |
| 9 hours |
Novo Nordisk, Vivtex to Develop Obesity Drugs in Deal Valued at Up to $2.1 Billion
NVO
The Wall Street Journal
|
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite