|
|
|

|
|||||

|
|
Sanofi SNY reported fourth-quarter 2025 adjusted earnings of 89 cents per American depositary share, which beat the Zacks Consensus Estimate of 84 cents. Earnings of €1.53 per share rose 16.8% on a reported basis and 26.7% on a constant currency rate (“CER”) basis, driven by cost control.
Net sales rose 7.0% on a reported basis to $13.2 billion (€11.3 billion). Sales rose 13.3% on a CER basis. Sales slightly missed the Zacks Consensus Estimate of $13.4 billion.
Sales rose 22.6% at CER in the United States, 8.0% in the Rest of the World (including China, Japan, Brazil and Russia) and 0.2% in Europe.
All growth rates mentioned below are on a year-on-year basis and at CER.
In Immunology, blockbuster drug, Dupixent generated sales of €4.25 billion in the quarter, up 32.2% year over year, driven by strong prescription trends across all approved indications and geographies.
Sales of the drug in the United States rose 35.9%. Dupixent sales rose 17.9% in Europe and 25.0% in the Rest of the World.
Sanofimarkets Dupixent in partnership with Regeneron REGN. While sales are recorded by Sanofi, Regeneron records its share of profits/losses in connection with global sales of Dupixent.
Among Sanofi’s rare disease drugs, the rare blood disorder drug, Altuviiio, a once-weekly new class of factor VIII therapy for hemophilia A, recorded sales of €324 million in the fourth quarter, up 53.0% year over year, mainly driven by patient switches in the U.S. hemophilia A market and launch in Japan and Taiwan. As much as 85% of Altuviiio’s sales were in the United States.
Among the rare disease drugs in the Pompe franchise, Nexviazyme/Nexviadzyme recorded sales of €203 million, up 15.8% year over year, mainly driven by strong sales growth in Europe. Myozyme sales declined 5.3% year over year to €122 million due to patients switching to Nexviazyme. Fabrazyme sales were €252 million, down 1.1% year over year. In the Gaucher franchise, Cerezyme sales rose 2.9% year over year to €171 million.
Cablivi recorded sales of €69 million, up 1.4% year over year, driven by patient growth in the United States and Europe.
Among the rare blood disorder drugs, Eloctate sales declined 16.0% to €63 million in the quarter due to patients switching to the new drug Altuviiio.
Xenpozyme recorded sales of €61 million in the quarter, up 65.8% year over year.
New drug Qfitlia (fitusiran), which was approved by the FDA last year in March for treating hemophilia A and B, generated €4 million in sales, the same as in the previous quarter.
Wayrilz (rilzabrutinib), which was approved for treating immune thrombocytopenia in the United States in August last year, generated €6 million in sales in the fourth quarter versus €1 million in the previous quarter.
All sales of Qfitlia and Wayrilz were in the United States in the fourth quarter, as the drugs are not yet approved in ex-U.S. markets.
Ayvakit, added from last year’s acquisition of Blueprint Medicines, recorded sales of €168 million compared with €137 million in the previous quarter, driven by continued patient growth.
In neurology medicines, Aubagio sales declined 30.8% year over year to €51 million due to the loss of exclusivity in 2023.
In Oncology, Sarclisa sales rose 27.7% year over year to €157 million, driven by market share gains globally and increased use in earlier lines of treatment.
In Others, Toujeo recorded sales of €332.0 million, up 18.6% year over year, driven mainly by higher sales in Rest of World. Lantus sales rose 1.1% to €419 million. Rezurock recorded sales of €113 million, down 6.1% year over year. Tzield sales were €16 million in the quarter, flat year over year.
Plavix sales rose 7.6% to €214 million. Lovenox sales decreased 21.6% to €179 million.
Total vaccine sales declined 2.5% to €2.04 billion, mainly due to lower sales of Sanofi and partner AstraZeneca’s AZN respiratory syncytial virus or RSV antibody, Beyfortusand Polio/Pertussis/Hib (PPH) vaccines.
Sales of flu vaccines rose 31.5% year over year to €575 million due to a higher vaccination rate than expected previously. Sales of PPH vaccines declined 9.5% year over year to €551 million. Sales of meningitis, travel and other endemic vaccines declined 4.8% year over year to €227 million in the quarter.
AstraZeneca-partnered Beyfortus recorded sales of €686 million in the quarter, down 14.9% year over yeardue to a tough comparison from inventory increase in the year-ago quarter.
Sanofi issued its financial outlook for 2026.
Sanofi expects sales to rise by a high single-digit percentage at CER. Sanofi expects earnings to grow faster than sales in 2026 at CER (before stock buyback). Overall, Sanofi expects to be able to sustain its profitable growth momentum in 2026.
In 2026, Sanofi expects to buy back stock worth €1 billion in 2026.
Sanofi’s fourth-quarter results were mixed as it beat estimates for earnings but missed the same for sales. Higher sales of Dupixent and contributions from new products like Altuviiio and Ayvakit drove top-line growth, offsetting lower sales of vaccines. However, influenza vaccines performed better than expected in the fourth quarter. Dupixent continues to be the key top-line driver with global sales rising above €4 billion for the second consecutive quarter. Sales of its newly launched drugs and vaccines rose almost 50% in the quarter. Sanofi’s 2026 outlook was decent, and the company said it expects continued profitable growth for the next five years.
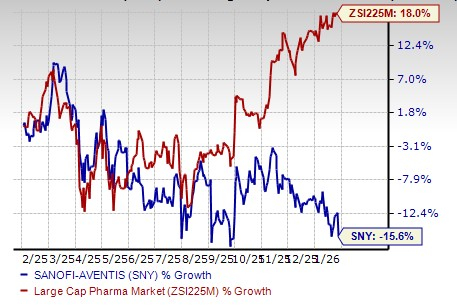
Sanofi’s shares were up around 1% in pre-market trading in response. Sanofi’s stock has declined 15.6% in the past year against the industry’s increase of 18.0%.
Sanofi is seeing a good uptake of its new medicines and vaccines. Altuviiio achieved blockbuster sales in 2025 and Ayvakit is expected to become the next blockbuster drug in 2026. Last year, Sanofi launched Qfitlia and Wayrilz. Some potential blockbuster assets in phase III development or under registration are amlitelimab, frexalimab and tolebrutinib. However, in December, the FDA issued a complete response letter to its new drug application, seeking approval for its BTK inhibitor, tolebrutinib, for treating non-relapsing secondary progressive multiple sclerosis. Regulatory applications for amlitelimab and frexalimab are planned in 2026 and 2027, respectively.
Sanofi has also been active on the M&A front. In December, it announced a definitive agreement to acquire vaccine maker, Dynavax Technologies DVAX for $15.50 per share in cash for a total equity value of approximately $2.2 billion. The acquisition will boost Sanofi’s adult vaccines franchise by adding DVAX’s marketed adult hepatitis B vaccine, Heplisav-B and a mid-stage shingles candidate. Last year, it acquired Blueprint Medicines, which has expanded its presence in rare immunological diseases and Vigil Neuroscience, which expanded its early-stage pipeline in neurology.
Sanofi currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-18 | |
| Feb-18 | |
| Feb-18 | |
| Feb-18 | |
| Feb-18 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-16 | |
| Feb-16 | |
| Feb-16 | |
| Feb-16 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite