|
|
|

|
|||||

|
|
Regeneron Pharmaceuticals, Inc. REGN reported fourth-quarter 2025 adjusted earnings per share (EPS) of $11.44, which comfortably beat the Zacks Consensus Estimate of $10.56. However, the bottom line was down 5% from $12.07 recorded in the year-ago quarter, primarily due to higher expenses.
Total revenues grew 3% year over year to $3.9 billion due to higher sales of Eylea HD and increased Dupixent profits. Revenues also beat the Zacks Consensus Estimate of $3.8 billion.
However, shares are trading down despite the outperformance, probably due to a decline in bottom line on a year-over-year basis.
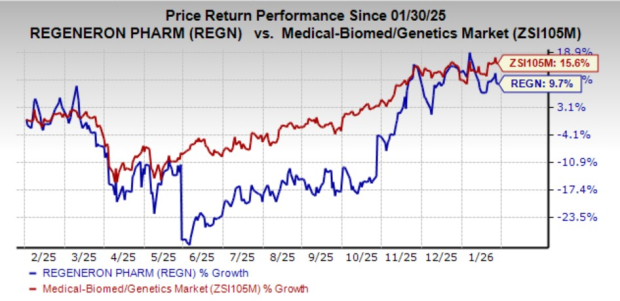
Regeneron’s shares have gained 9.7% in the past year compared with the industry’s growth of 15.6%.
Lead drug, Eylea, is approved for various ophthalmology indications (neovascular age-related macular degeneration, diabetic macular edema and macular edema, among others).
Eylea’s sales in the United States plunged 52% year over year to $577 million, primarily due to increased competition from other drugs like Roche’s RHHBY Vabysmo, loss in market share to compounded bevacizumab due to patient affordability constraints and transition of patients to higher doses of the drug (Eylea HD).
Eylea sales in the United States also fell short of the Zacks Consensus Estimate of $592 million.
Please note that Regeneron co-developed Eylea with the HealthCare unit of Bayer AG BAYRY. Regeneron records net product sales of Eylea and Eylea HD in the United States and Bayer does the same outside the country. The company records its share of profits in connection with Eylea and Eylea HD sales outside the United States within collaboration revenues.
In August 2023, the FDA approved Eylea HD (higher dose of Eylea) for the treatment of patients with wet age-related macular degeneration, diabetic macular edema and diabetic retinopathy.
Eylea HD generated revenues of $506 million in the United States, up 66% year over year, due to higher sales volumes driven by increased demand. Eylea HD sales beat the Zacks Consensus Estimate of $477 million.
Total revenues include collaboration revenues of $2 billion from Sanofi SNY and Bayer. The figure increased 22.6% from that recorded in the year-ago quarter. Total collaboration revenues beat the Zacks Consensus Estimate of $1.9 billion.
Sanofi’s collaboration revenues increased 35% to $1.64 billion, driven by profits associated with higher Dupixent sales. The figure beat the Zacks Consensus Estimate of $1.6 billion. We note that Sanofi records global net product sales of Dupixent and Kevzara, while Regeneron records its share of profits/losses in connection with the global sales of both drugs within collaboration revenues. Dupixent’s sales increased 34% year over year to $4.9 billion.
Bayer’s collaboration revenues totaled $319 million, down 15% year over year.
Regeneron records net product sales of Praluent in the United States and Sanofi does the same outside the country. SNY pays REGN a royalty on such sales. Regeneron records global net product sales of Libtayo and pays Sanofi a royalty on such sales.
Total Libtayo sales were $425 million, up 16% year over year. The figure, however, missed the Zacks Consensus Estimate of $482 million.
Praluent’s net sales in the United States were $73 million.
Gross margin on net product sales decreased to 85% from 86% due to ongoing investments to support the manufacturing operations.
Adjusted R&D expenses increased 9% year over year to $1.3 billion due to the advancement of the company's pipeline. Adjusted SG&A expenses increased 1% to $691 million.
In February 2025, the board of directors authorized a new share repurchase program to repurchase up to an additional $3.0 billion of the common stock. During the fourth quarter of 2025, REGN repurchased shares for $671 million. As of Dec. 31, 2025, approximately $1.5 billion remained available for share repurchases.
Revenues increased 1% year over year to $14.34 billion. Adjusted EPS in 2025 was $44.31, down 3% from $45.62 in 2024.
In November 2025, the European Commission (EC) approved Dupixent for the treatment of chronic spontaneous urticaria in adults and adolescents aged 12 years and older who remain symptomatic despite antihistamine treatment.
Eylea HD continues to build regulatory and commercial momentum. In November 2025, the FDA approved Eylea HD for the treatment of retinal vein occlusion (RVO), with up to every 8-week dosing following an initial monthly loading phase. Importantly, the FDA also approved an every four-week dosing option across all major indications — including wet age-related macular degeneration, diabetic macular edema, diabetic retinopathy, and RVO — providing physicians with greater flexibility and supporting broader market adoption.
Earlier this month, the EC approved Eylea 8 mg (marketed as Eylea HD in the United States) for RVO.
In October and November 2025, the FDA and EC, respectively, approved Libtayo as an adjuvant treatment for adults with cutaneous squamous cell carcinoma (CSCC) at high risk of recurrence following surgery and radiation. This approval makes Libtayo the first and only immunotherapy authorized in the adjuvant setting for CSCC, significantly expanding its addressable market beyond metastatic or locally advanced disease.
REGN’s collaboration agreement with Tessera Therapeutics, Inc. became effective this month. The deal aims to develop and commercialize TSRA-196, Tessera’s investigational gene-editing therapy for alpha-1 antitrypsin deficiency (AATD). Under the agreement, Tessera will lead the initial first-in-human study, while Regeneron will assume responsibility for subsequent global development and commercialization.
Regeneron’s fourth-quarter performance was encouraging, with overall revenues rising despite continued declines in sales of its flagship product, Eylea. This performance underscores the resilience and breadth of the company’s diversified portfolio.

Regeneron Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Regeneron Pharmaceuticals, Inc. Quote
Eylea sales remain under pressure amid intensifying competition from Roche’s Vabysmo, which has seen strong and rapid uptake. Vabysmo was designed to inhibit both Ang-2 and VEGF-A pathways, offering a differentiated mechanism that has resonated with physicians.
Regeneron is actively strengthening its oncology portfolio as well to further diversify its revenue base. The company’s oncology franchise recently gained momentum following the label expansion of Libtayo, helping offset headwinds in the ophthalmology segment.
Regeneron currently carries a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-16 | |
| Feb-16 | |
| Feb-15 | |
| Feb-14 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-12 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite