|
|
|

|
|||||

|
|
New Feature: See Wall Street analyst ratings directly on Finviz charts for deeper context into price action.
GRAIL, Inc. GRAL announced the submission of the final module of its premarket approval application to the FDA for the Galleri multi-cancer early detection (MCED) test. The FDA had previously granted the test Breakthrough Device designation in 2018, reflecting its potential to improve early cancer detection.
The PMA submission focused on the United States-based PATHFINDER 2 study data with one year of follow-up and from the prevalent screening round (first year) of the NHS-Galleri trial.
Management highlighted that cancer is the leading cause of death among adults above the age of 50 in the United States, with many high-mortality cancers diagnosed at advanced stages. Adding Galleri alongside standard single-cancer screening tests could improve cancer detection rates and help address gaps in the current screening paradigm, particularly for cancers with no routine screening options. Galleri has been evaluated across multiple study types and the FDA submission has reached a milestone toward broader access and potential public health impact.
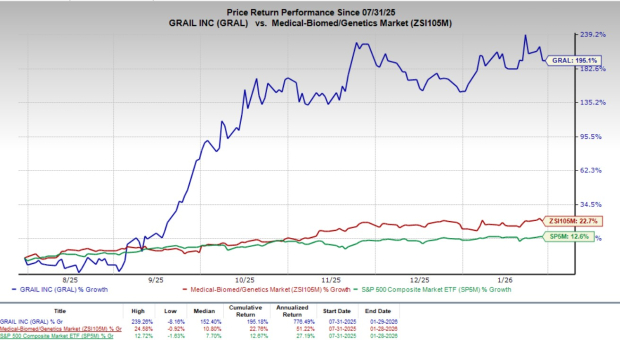
Following the announcement, GRAL shares remained almost flat at yesterday’s closing. Over the past six months, shares of the company have skyrocketed 195.1% compared with the industry’s 22.7% growth and the S&P 500’s 12.6% rise.
In the long run, FDA approval would be a transformative catalyst for GRAIL, enabling broader clinical adoption of the Galleri test and its integration into population-level cancer screening programs. The robust clinical evidence from U.S. and U.K. studies enhances regulatory credibility and de-risks commercialization. Combined with Breakthrough Device designation, this development positions GRAIL as a leader in multi-cancer early detection, with the potential for sustained revenue growth and increased value within the oncology diagnostics market.
GRAL currently has a market capitalization of $3.94 billion.
The NHS-Galleri trial is conducted in collaboration with the NHS in England to evaluate the clinical utility and performance of the Galleri test when used alongside standard population screening. The trial enrolled more than 140,000 asymptomatic individuals aged 50-77, who provided three blood samples over a two-year period at annual intervals. The primary endpoint is a reduction in stage III-IV cancer diagnoses among individuals receiving the Galleri test versus standard care alone. The outcome will be assessed across three relevant cancer groups, focusing on 12 pre-specified cancers that account for two-thirds of cancer-related deaths in England and the United States. Secondary endpoints include reductions in stage IV cancer, Galleri test’s performance metrics in cancer detection rates, safety profile and its impact on the use of healthcare resources.
The PATHFINDER 2 study is designed to assess the safety and performance of the Galleri MCED test in 35,000 participants aged 50 and older who are eligible for cancer screening. The study’s primary endpoints focus on the test performance based on the type and number of follow-up diagnostic procedures among participants with a cancer signal detected result.
It also assesses positive and negative predictive values, sensitivity, specificity and accuracy of CSO prediction. Participants with a cancer signal detected result undergo additional diagnostic workups guided by the predicted CSO. Secondary endpoints include assessing the impacts of Galleri on the use of standard cancer screening procedures, as well as participant-reported outcomes over time, including anxiety levels and satisfaction with the test.
The PMA submission includes safety and performance data from 25,490 consented participants in the United States-based PATHFINDER 2 study with one year of follow-up, along with results from the first screening round of the NHS-Galleri trial. The submission includes a bridging analysis linking the performance of the Galleri version used in PATHFINDER 2 and the NHS-Galleri trial with the updated version submitted to the FDA for premarket approval, helping ensure continuity of performance evidence.
Going by data provided by Precedence Research, the cancer diagnostics market was valued at $170 billion in 2025 and is expected to witness a CAGR of 8.6% through 2034. Factors like the growing number of cancer patients across the world, the benefits of early cancer diagnosis in reducing mortality rate in the United States, high per-capita health care expenditure of countries like the United States, Germany and Japan, and heavy investment by the private firms are driving the market’s growth.
GRAIL reported positive long-term follow-up data from the SYMPLIFY study, reinforcing the clinical value of its Galleri multi-cancer early detection test in symptomatic patients. Extended registry results showed that many cases initially classified as false positives were later confirmed as cancer, driving a positive predictive value of 84.2%.
The company announced positive safety and performance data from its PATHFINDER 2 study presented at ESMO 2025, highlighting Galleri’s ability to detect cancers at early stages in a large screening population. The results support Galleri’s potential to address cancers lacking routine screening options and strengthen its clinical positioning.
GRAIL announced a collaboration with Samsung to commercialize the Galleri test in Korea and other Asian markets, including Japan and Singapore. The partnership enhances GRAIL’s international expansion strategy and is supported by Samsung’s planned $110-million equity investment, strengthening GRAIL’s financial position and regional footprint.

GRAIL, Inc. price | GRAIL, Inc. Quote
Currently, GRAIL carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the broader medical space are Veracyte VCYT, AtriCure ATRC and Boston Scientific BSX.
Veracyte, sporting a Zacks Rank #1 (Strong Buy) at present, reported third-quarter 2025 adjusted earnings per share (EPS) of 51 cents, which surpassed the Zacks Consensus Estimate by 59.4%. Revenues of $131.8 million beat the Zacks Consensus Estimate by 5.5%. You can see the complete list of today’s Zacks #1 Rankstocks here.
VCYT has an estimated earnings recession rate of 3% for 2026 compared with the industry’s 17.7% rise. The company beat earnings estimates in the trailing four quarters, the average surprise being 45.1%.
AtriCure, currently carrying a Zacks Rank #2 (Buy), reported a third-quarter 2025 adjusted loss of 1 cent per share, surpassing the Zacks Consensus Estimate by 90.9%. Revenues of $134.3 million beat the Zacks Consensus Estimate by 2.1%.
ATRC has an estimated earnings growth rate of 91.7% for 2026 compared with the industry’s 17.2% rise. The company beat earnings estimates in the trailing four quarters, the average surprise being 67.1%.
Boston Scientific, currently carrying a Zacks Rank #2, reported a third-quarter 2025 adjusted EPS of 75 cents, which surpassed the Zacks Consensus Estimate by 5.6%. Revenues of $5.07 billion beat the Zacks Consensus Estimate by 1.9%.
BSX has an estimated long-term earnings growth rate of 16.4% compared with the industry’s 13.1% rise. The company’s earnings beat estimates in the trailing four quarters, the average surprise being 7.4%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 12 min | |
| 53 min |
Stock Market Today: Dow Turns Higher As Supreme Court Nixes Trump Tariffs (Live Coverage)
GRAL -48.57%
Investor's Business Daily
|
| 1 hour | |
| 1 hour | |
| 2 hours | |
| 2 hours | |
| 2 hours | |
| 4 hours | |
| 6 hours | |
| 7 hours | |
| 7 hours | |
| 8 hours | |
| 12 hours | |
| Feb-19 | |
| Feb-19 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite