|
|
|

|
|||||

|
|
New Feature: See Wall Street analyst ratings directly on Finviz charts for deeper context into price action.
Eli Lilly and Company’s LLY stock has declined 5.1% so far this month, despite announcing strong second-quarter results on Aug. 7. The company beat estimates for both earnings and sales. Sales of Lilly’s key drugs, Mounjaro, Zepbound, Jardiance and Trulicity beat estimates. Lilly’s new products also contributed to sales growth.
The company also raised its financial outlook for the year, backed by a strong performance in the first half, mainly driven by the robust growth of Mounjaro and Zepbound.
However, the much-awaited data from a phase III study on Lilly’s oral GLP-1 candidate, orforglipron for obesity, failed to impress investors. The ATTAIN-1 study met the primary endpoint for orforglipron, showing superior body weight reduction compared to placebo for all three doses tested (6 mg, 12 mg and 36 mg). The highest dose (36 mg) of orforglipron delivered a weight loss of 12.4% at 72 weeks, which fell short of investors’ expectations, causing LLY’s stock to crash 14% on Aug. 7.
Investors were probably expecting orforglipron to match rival Novo Nordisk’s NVO Wegovy’s average weight loss in a range of 13-15% or even exceed that percentage. Patient discontinuation rates in the study were also considered high, with 24.4% of patients in the 36 mg dose arm discontinuing treatment. Orforglipron, an oral pill, is being viewed as a key candidate in Lilly's obesity pipeline, which has the potential to transform the treatment space as both Zepbound and Wegovy are weekly injections.
However, LLY’s stock has somewhat recovered from the lows it witnessed after its second-quarter results, leading many to question if the stock’s decline in response to the obesity data was an overreaction.
Let’s understand the company’s strengths and weaknesses to better analyze how to play the stock amid the recent price decline.
Lilly boasts a robust portfolio of treatments for diabetes and other cardiometabolic conditions, with its cardiometabolic division emerging as the company’s strongest segment. This success is largely attributed to its widely used GLP-1 therapies — Mounjaro for diabetes and Zepbound for weight loss.
Despite being on the market for only around three years, Mounjaro and Zepbound have become key top-line drivers for Lilly, with demand rising rapidly. Mounjaro and Zepbound account for around 50% of the company’s total revenues.
Though sales of Mounjaro and Zepbound were below expectations in the second half of 2024, hurt by slower-than-expected growth and unfavorable channel dynamics, their sales picked up in the first half of 2025, driven by launches of the drugs in new international markets and improved supply from ramped-up production. The positive trend is expected to continue through the rest of the year.
In the first half of 2025, Lilly produced more than 1.6x the amount of salable incretin doses when compared to the first half of 2024, helped by its significant step-up in capacity. Lilly expects to bring more capacity online in the second half of 2025 and expects its production capabilities to increase further. Lilly expects to produce at least 1.8x the number of salable incretin doses in the second half of 2025 compared to the second half of 2024.
Lilly continues to launch Mounjaro in new countries like Mexico and Brazil. Lilly has now launched Mounjaro in most major markets. The initial launch uptake in Mexico, Brazil, China and India has been excellent, the company said on the second-quarter conference call.
Regulatory approvals for new indications are expected to further boost sales. In late December 2024, the FDA approved Zepbound for a second indication — moderate-to-severe obstructive sleep apnea in adults with obesity. Based on positive data from a phase III cardiovascular outcome study, Lilly plans to submit global regulatory applications to support a label cardiovascular indication by the end of 2025. Lilly is also conducting a phase III study for type 1 diabetes. A phase II study in metabolic dysfunction-associated steatohepatitis (MASH) met its primary endpoint in 2024.
In 2025, Lilly launched additional Zepbound lower-priced vial doses and offered new savings for self-pay patients to boost sales. Lilly is also expanding its incretin production capability.
In addition to Mounjaro and Zepbound, Lilly has secured approvals for several other new therapies over the past few years. These include Omvoh for treating ulcerative colitis and Crohn’s disease, BTK inhibitor Jaypirca for mantle cell lymphoma and chronic lymphocytic leukemia, Ebglyss for moderate-to-severe atopic dermatitis, and Kisunla (donanemab) for early symptomatic Alzheimer’s disease. These newly approved drugs are also contributing to Lilly’s revenue growth.
Lilly expects its new drugs, Mounjaro, Zepbound, Omvoh, Jaypirca, Ebglyss and Kisunla, along with the expanded use of existing drugs, to drive sales growth in the second half of 2025. It also expects the potential launch of new medicines like imlunestrant for metastatic breast cancer to contribute to growth in 2025.
The company is also making rapid pipeline progress in obesity, diabetes and cancer. Lilly is investing broadly in obesity and has several new molecules currently in clinical development. This includes two late-stage candidates, orforglipron, and triple-acting incretin, retatrutide (which combines GLP-1, GIP and glucagon), and some mid-stage candidates, bimagrumab, eloralintide and mazdutide.
Lilly plans to file regulatory applications for orforglipron in obesity in the fourth quarter of 2025 and for type II diabetes in the first half of 2026. It also expects the first data readout from a phase III study on retatrutide in osteoarthritis of the knees later in 2025.
LLY is also working to diversify beyond GLP-1 drugs by expanding into cardiovascular, oncology, and neuroscience areas. In 2025, it announced three M&A deals. It acquired Verve Therapeutics this month to add gene therapies for heart disease to its pipeline. Lilly has also entered into agreements to acquire Scorpion Therapeutics’ oncology drug and SiteOne Therapeutics’ non-opioid pain candidate.
The obesity market is expected to expand to $100 billion by 2030, according to data from Goldman Sachs, which means fierce competition is inevitable. Lilly and Novo Nordisk presently dominate the market.
Mounjaro and Zepbound face strong competition from Novo Nordisk’s semaglutide medicines, Ozempic for diabetes and Wegovy for obesity.
Several companies like Amgen AMGN and Viking Therapeutics VKTX are also making rapid progress in the development of more potent and convenient GLP-1-based candidates in their clinical pipeline.
Amgen is developing MariTide, a GIPR/GLP-1 receptor, as a single dose in a convenient autoinjector device with a monthly and maybe less frequent dosing. In March, Amgen initiated two phase III studies on MariTide in obesity as part of its comprehensive MARITIME phase III program. Since June, Amgen has initiated two more phase III studies in other obesity related conditions.
Viking Therapeutics’ dual GIPR/GLP-1 receptor agonist, VK2735, is being developed both as oral and subcutaneous formulations for the treatment of obesity. Phase III obesity studies with the subcutaneous formulation of VK2735 were recently initiated, while the oral formulation is being evaluated in a phase II study.
NVO, LLY and VKTX are racing to introduce oral weight-loss pills, as Wegovy and Zepbound are both injectable drugs. Novo Nordisk has already filed a new drug application (NDA) for an oral version of Wegovy and also has several next-generation candidates in its obesity pipeline, like CagriSema (a combination of semaglutide and cagrilintide) and an oral pill, amycretin (a dual GLP-1 and amylin receptor agonist). The FDA is expected to decide on the Wegovy oral formulation NDA in the fourth quarter.
Lilly is battling several challenges at present. Sales of its key medicine, Trulicity, are declining in the United States due to competitive dynamics, including Mounjaro switches and supply constraints. Prices of most of Lilly’s products are declining in the United States, including Mounjaro and Zepbound, primarily due to changes to estimates for rebates and discounts. Lilly’s U.S. net price has declined every year since 2021. In 2025, Lilly expects a mid-to-high single-digit percentage price decline, including U.S. Part D changes.
Rapid progress in the development of competing therapies in the GLP-1 market has also hurt the stock price. Effective July 1, CVS Caremark, a major pharmacy benefit manager, began excluding Zepbound from its preferred drug list while retaining NVO’s Wegovy. This decision hurt Zepbound prescriptions during July, and Lilly expects it to be a headwind to the rate of volume growth in the third quarter.
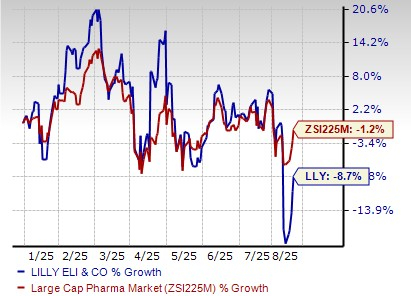
Lilly’s stock has declined 8.7% so far this year compared with the industry’s decrease of 1.2%.
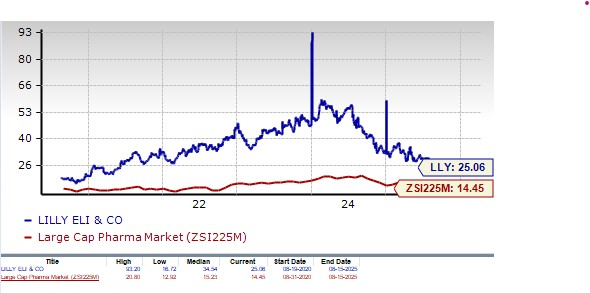
From a valuation standpoint, Lilly’s stock is expensive. Going by the price/earnings ratio, the company’s shares currently trade at 25.06 forward earnings, higher than 14.45 for the industry. However, the stock is trading below its 5-year mean of 34.54.
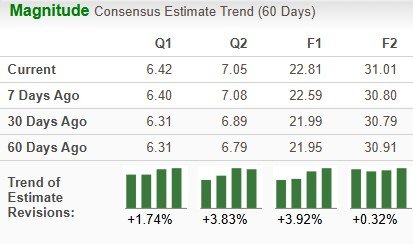
Estimates for Lilly’s 2025 earnings have risen from $21.99 to $22.81 per share in the past 30 days, while those for 2026 have risen from $30.79 to $31.01 over the same timeframe.
Lilly’s tremendous success with Mounjaro and Zepbound has made it the largest drugmaker with a market cap of more than $600 billion. No doubt, the weight-loss pill data was a disappointment. Many investors feel that the potential market for orforglipron is now less than previously expected. However, some investors feel the market reaction to the data was overblown and perceive that orforglipron may be used for maintenance therapy after patients lose weight with injectable products like Zepbound or Wegovy, with the potential to expand into other disease areas. There is significant demand for single oral pills for weight loss that can be manufactured at scale, and orforglipron has the potential to be approved.
In 2025, Lilly expects to record revenues in the range of $60.0 billion to $62.0 billion, indicating an impressive year-over-year growth of more than 30%
Despite an expensive valuation, recent price depreciation and rising competitive pressure, we suggest investors who own this Zacks Rank #3 (Hold) company retain it as it has solid growth prospects and a promising portfolio of new drugs and pipeline candidates. Consistently rising estimates also reflect investors’ optimistic outlook for the stock. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 |
Hims & Hers Agrees To Buy Eucalyptus In $1.15 Bil Deal. Stock Rallies.
NVO
Investor's Business Daily
|
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite