|
|
|

|
|||||

|
|
Regeneron Pharmaceuticals, Inc. REGN announced updated data on its investigational gene therapy DB-OTO from the CHORD study.
This gene therapy is being evaluated for profound genetic hearing loss due to variants of the otoferlin (OTOF) gene.
The latest data was presented during an oral presentation at the annual American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNSF) meeting. It was also published in The New England Journal of Medicine.
DB-OTO is an investigational cell-selective, dual adeno-associated virus (AAV) vector gene therapy designed to provide durable, physiological hearing to individuals with profound, congenital hearing loss caused by variants of the OTOF gene.
The CHORD is a registrational phase I/II multicenter, open-label study to evaluate the safety, tolerability and efficacy of DB-OTO in infants, children and adolescents with OTOF-related hearing loss. The study is currently enrolling children (<18 years of age) across sites in the United States, United Kingdom, Spain and Germany.
The study is being conducted in two parts. In the initial dose-escalation cohort (Part A), participants receive a single intracochlear infusion of DB-OTO in one ear. In the expansion cohort (Part B), participants receive DB-OTO in both ears at the selected dose from Part A.
Per REGN, the surgical procedure to administer DB-OTO leverages an approach similar to cochlear implantation, which enables use in infants.
Nine of the 12 participants (aged 10 months to 16 years) received the gene therapy unilaterally (in one ear) and three received it bilaterally (in both ears).
Results showed 11 out of 12 participants experienced clinically meaningful hearing improvements, including three who achieved normal hearing levels. Additionally, eight participants with longer follow-up showed stability or continued improvement in their hearing.
Among the three who completed speech assessments, all showed substantial improvement, with one able to identify one- and two-syllable words with no visual cues and respond to distant sounds and speech in noisy environments.
The trial met the primary endpoint with nine participants experiencing hearing improvements at a threshold of ≤70 decibel hearing level (dBHL) as assessed by behavioral pure tone audiometry (PTA) at week 24.
Hearing improvements remained stable or continued to improve in eight participants who had follow-up visits of ≥36 weeks (up to 72 weeks).
Both the surgical procedure and DB-OTO were well tolerated across all 12 participants.
DB-OTO received Orphan Drug, Rare Pediatric Disease, Fast Track and Regenerative Medicine Advanced Therapy designations from the FDA. The European Medicines Agency also granted the Orphan Drug Designation.
REGN targets regulatory submissions for DB-OTO in the United States later this year, pending discussions with the FDA.
REGN is making efforts to diversify its portfolio as the lead drug Eylea is under pressure due to competition from Roche’s RHHBY Vabysmo.
Eylea, an anti-vascular endothelial growth factor inhibitor (VEGF), is approved for various ophthalmology indications. The drug is the biggest contributor to the top line.
The uptake of Roche’s Vabysmo has been phenomenal. Roche has designed Vabysmo to block pathways involving Ang-2 and VEGF-A.
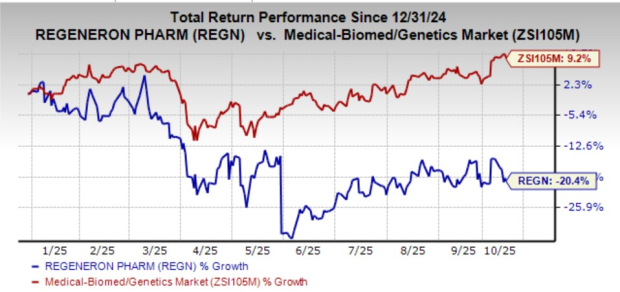
REGN stock has lost 20.4% year to date against the industry’s growth of 9.2%.
To counter the decline in Eylea sales, Regeneron has developed a higher dose of the drug. Eylea HD sales in the United States surged 29% in the second quarter due to higher sales volumes driven by increased demand.
REGN’s top line also comprises its share of profits/losses in connection with the global sales of Dupixent. Partner Sanofi SNY records global net product sales of Dupixent.
Solid sales of Dupixent (approved for use in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis) have fueled the top line for Sanofi and Regeneron.
The company’s oncology franchise received a boost with the recent FDA approval of a label expansion for its PD-1 inhibitor, Libtayo (cemiplimab-rwlc), as an adjuvant treatment for adult patients with cutaneous squamous cell carcinoma who are at high risk of recurrence after surgery and radiation.
The regulatory body had earlier approved linvoseltamab-gcpt for the treatment of relapsed or refractory (R/R) multiple myeloma (“MM”). The drug was granted accelerated approval by the FDA under the brand name Lynozyfic. It is also approved in the European Union to treat adults with R/R MM after at least three prior therapies, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody.
The approval of Ordspono (odronextamab) for treating adult patients with R/R follicular lymphoma or R/R diffuse large B-cell lymphoma after two or more lines of systemic therapy has also strengthened its oncology franchise.
However, the FDA recently issued a complete response letter for the BLA for odronextamab.
REGN currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 10 hours | |
| 11 hours | |
| 11 hours | |
| 13 hours | |
| Feb-16 | |
| Feb-16 | |
| Feb-15 | |
| Feb-14 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-12 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite