|
|
|

|
|||||

|
|
New Feature: See Wall Street analyst ratings directly on Finviz charts for deeper context into price action.
AbbVie Inc. ABBV reported third-quarter 2025 adjusted EPS of $1.86, which beat the Zacks Consensus Estimate of $1.77. The reported figure also exceeded the company’s guidance of $1.74-$1.78 issued earlier this month. Earnings declined 38.0% year over year, mainly due to higher IPR&D charges incurred during the quarter.
ABBV’s revenues of $15.78 billion beat the Zacks Consensus Estimate of $15.59 billion. Sales rose 9.1% year over year on a reported basis and 8.4% on an operational basis. This reported figure also surpassed the company’s forecast of $15.5 billion.
Revenues in the quarter were driven by robust sales of key drugs Rinvoq, Skyrizi, Venclexta and Vraylar, coupled with significant contributions from newer drugs, namely Ubrelvy, Qulipta, Elahere and Vyalev. Sales of Humira and Imbruvica declined year over year.
All growth rates mentioned below are on a year-on-year basis and at constant exchange rates (CER).
In immunology, net revenues from Rinvoq for the quarter totaled $2.18 billion, up 34.1%. The upside was likely driven by market share gains across all approved indications, as well as the recent label expansion in giant cell arteritis across the United States and EU in April. Rinvoq’s sales beat the Zacks Consensus Estimate and our model estimate, both pegged at $2.16 billion.
Net revenues recorded from Skyrizi were $4.71 billion, up 46.0%. This surge in sales was likely driven by strong volume growth and continued market share gains. Skyrizi sales beat both the Zacks Consensus Estimate of $4.56 billion and our model estimate of $4.54 billion.
AbbVie’s flagship product Humira recorded a sales decline of 55.7% to $993 million for the third quarter. Sales in the United States declined 65% to $619 million, while ex-U.S. market sales were down 20.5% to $374 million. The drug’s overall sales missed the Zacks Consensus Estimate of $1.15 billion and our model estimate of $1.16 billion.
This substantial decline in Humira sales was due to the drug’s loss of exclusivity in the United States since January 2023. The drug lost its exclusivity in ex-U.S. territories following the launch of generics in 2018.
Sales from the neuroscience portfolio increased 19.6% to $2.84 billion, driven by higher sales of Botox Therapeutic, depression drug Vraylar, and migraine drugs Ubrelvy and Qulipta. Neuroscience sales beat the Zacks Consensus Estimate and our model estimate of $2.74 billion and $2.73 billion, respectively.
While Botox Therapeutic sales rose 15.8% to $985 million, sales of Vraylar increased 6.7% to $934 million.
Sales of Ubrelvy totaled $354 million, up 31.5%. Qulipta sales increased 63.1% to $288 million.
Sales of Vyalev, the recently approved transformative therapy for advanced Parkinson’s disease, totaled $138 million compared with $98 million in the previous quarter.
Sales from the oncology franchise fell 1.3% to $1.68 billion in the quarter. This slight downtick was due to declining Imbruvica sales, which more than offset the growth in sales of newer oncology drugs, Epkinly and Elahere, as well as rising Venclexta sales. The metric missed both the Zacks Consensus Estimate and our model estimate of $1.69 billion and $1.71 billion, respectively.
Third-quarter net revenues from Imbruvica totaled $706 million, down 14.8%. Though this figure beat our model estimate of $703 million, it missed the Zacks Consensus Estimate of $708 million. ABBV markets this drug in partnership with Johnson & Johnson JNJ.
U.S. sales of J&J-partnered Imbruvica declined 17.9% to $507 million due to rising competition from novel oral treatments. AbbVie shares international profits earned from Imbruvica with J&J. The company’s share of profit from the drug’s international sales declined 5.8% to $199 million.
AbbVie’s leukemia drug Venclexta generated revenues of $726 million in the reported quarter, reflecting 4.9% growth. The company markets Venclexta in collaboration with Roche RHHBY. While sales from the Roche-partnered drug beat the Zacks Consensus Estimate of $712 million, it missed our model estimate of $727 million.
Sales of the breast cancer drug, Elahere, rose 22.4% to $170 million. However, the metric missed both the Zacks Consensus Estimate of $199 million and our model estimate of $197 million.
Epkinly sales, which comprise AbbVie’s share of profit from U.S. revenues and product revenues from international markets, amounted to $69 million in the quarter compared with $70 million in the previous quarter. The drug is marketed in partnership with Genmab GMAB.
AbbVie’s aesthetics portfolio sales were down 4.2% to $1.19 billion, which missed the Zacks Consensus Estimate of $1.27 billion and our model estimate of $1.30 billion. Botox Cosmetic sales fell 5.4% to $637 million. Juvederm sales declined 3.2% to $253 million.
Eye care portfolio sales declined 4.2% to $509 million. Sales of Ozurdex, a key drug in the portfolio, fell 4.0% to $117 million.
Adjusted SG&A expenses rose 2.6% year over year to $3.41 billion. Adjusted R&D expenses amounted to $2.26 billion, up 9.8%.
AbbVie raised its EPS guidance for the full year. The company expects adjusted EPS to be in the range of $10.61-$10.65, up from the previous guidance of $10.38-$10.58.
AbbVie posted better-than-expected Q3 results and also raised its EPS guidance for 2025, likely driven by stronger-than-expected sales of its newer immunology drugs, Rinvoq and Skyrizi. The company’s top line also benefited from solid growth in the neuroscience franchise, supported by newer assets like Ubrelvy, Qulipta and Vyalev. However, overall growth was partially offset by Humira’s continued generic erosion and weaker aesthetics sales.
Shares of AbbVie were trading lower in pre-market today, likely due to the softer sales performance of its aesthetics portfolio, which fell short of expectations. We had previously expected the company to make a recovery in this segment, but sales instead declined as demand for facial injectables remained muted.
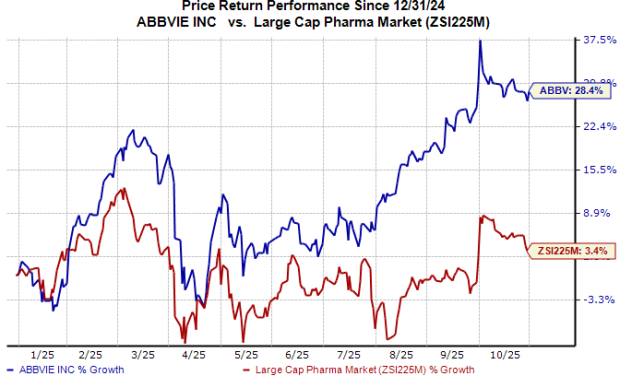
Year to date, the stock has gained 28% compared with the industry’s 3% growth.
To offset the declines from its legacy products, AbbVie is strengthening its long-term growth platform through M&A activity and internal innovation. In May, AbbVie secured approval for Emrelis, its first internally developed solid tumor therapy, marking a key milestone in its oncology expansion.
Last month, AbbVie submitted two regulatory filings with the FDA — one for pivekimab sunirine (PVEK) to treat a rare and aggressive blood cancer called blastic plasmacytoid dendritic cell neoplasm (BPDCN) and another for tavapadon in early Parkinson’s disease. Both of these drugs were added through prior acquisitions, and together with Emrelis, they reflect AbbVie’s accelerating focus on expanding its oncology and neuroscience footprint. The company has completed more than 30 M&A transactions since the beginning of 2024, underscoring its proactive approach to pipeline diversification and portfolio renewal aimed at driving sustainable long-term growth.

AbbVie Inc. price | AbbVie Inc. Quote
AbbVie currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 2 hours | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-20 | |
| Feb-19 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite