|
|
|

|
|||||

|
|
Vertex Pharmaceuticals VRTX reported adjusted earnings of $4.80 per share for the third quarter of 2025, surpassing the Zacks Consensus Estimate of $4.55. Earnings rose around 10% year over year.
Total revenues of $3.08 billion beat the Zacks Consensus Estimate of $3.04 billion. Total revenues rose 11% year over year, primarily driven by higher sales of Trikafta/Kaftrio and contributions from its three new drugs, Alyftrek, Journavx and Casgevy.
Its total revenues rose 15% year over year in the United States to $1.98 billion, driven by strong demand, favorable net pricing compared with the prior year and contributions from new products. Outside the U.S. market, sales increased 4% to $1.10 billion, driven by strong CF growth and contribution from Casgevy
Trikafta generated sales worth $2.65 billion, up 2.6% year over year. The product’s sales beat the Zacks Consensus Estimate as well as our model estimate of $2.56 billion.
Alyftrek, a next-in-class triple combination regimen for CF, generated sales worth $247.0 million in the third quarter compared with $156.8 million in the second quarter. Vertex said that the U.S. launch of Alyftrek is progressing well across all patient groups while in ex-U.S. markets, the early launch of Alyftrek is off to a strong start in multiple European countries.
Revenues from other products declined 6% year over year to $175.8 million. This included revenues from Vertex and partner CRISPR Therapeutics’ CRSP one-shot gene therapy, Casgevy, sales from Vertex’s new pain drug, Journavx, as well as sales of VRTX’s other CF products, Symdeko/Symkevi (marketed as Symkevi in Europe), Orkambi and Kalydeco.
Casgevy sales of $16.9 million declined 44.4% on a sequential basis. VRTX and CRSP’s new drug, Casgevy, is approved for two blood disorders, sickle cell disease and transfusion-dependent beta-thalassemia.
Vertex expects over $100 million in Casgevy revenues this year and significant growth in 2026. Vertex recorded $61.5 million in Casgevy revenues in the first nine months of 2025.
Journavx (suzetrigine) generated $19.6 million in sales in the third quarter compared with $12 million in the second quarter. Journavx, a novel non-opioid pain medicine (suzetrigine), was approved in the United States in January.
Adjusted research and development (R&D) expenses rose 12.6% year over year to $861.1 million to support its pipeline development.
Adjusted selling, general and administrative (SG&A) expenses rose 23% to $369.0 million in the reported quarter to support the launch of Journavx.
During the third quarter, Vertex recorded acquired in-process research and development (AIPR&D) costs of $54.5 million compared with $15.0 million in the year-ago quarter.
Adjusted operating income rose 6% to $1.38 billion in the quarter.
The company tightened its total revenue guidance for full-year 2025 from a range of $11.85-$12 billion to $11.9 to $12.0 billion.
The revenue range indicates growth of 8-9%, to be driven by continued CF franchise growth, including contribution from Alyftrek, over $100 million of Casgevy revenues and increased contribution from Journavx in the fourth quarter as it sees rising volumes.
Combined adjusted R&D, AIPR&D and SG&A expense guidance for 2025 was raised from a range of $4.9-$5 billion to $5.0 to $5.1 billion. The guidance was increased to account for higher expenses to support Journavx’s launch and for the acceleration of the povetacicept pipeline program.
The adjusted tax rate was reduced from a range of 20.5%-21.5% to 17% to 18%.
Vertex’s third-quarter results were strong as it beat estimates for both earnings and sales. However, shares of Vertex declined 4% in after-hours trading on Monday, probably due to the soft sales performance of Casgevy, which indicates that its uptake may be slower than expected.
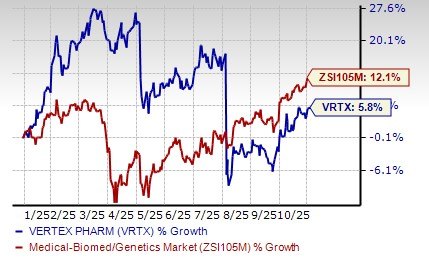
Shares of Vertex have risen 5.8% so far this year compared with the industry’s rise of 12.1%.
Vertex’s CF sales continue to grow, driven by higher sales of Trikafta/Kaftrio. Regarding the performance of its newly launched products, the uptake of Alyftrek is strong, while Journavx launch metrics and early reimbursement progress look favorable. Casgevy’s launch has been slow. Vertex also has a rapidly advancing mid to late-stage pipeline in other disease areas, like acute and peripheral neuropathic pain, APOL1-mediated kidney disease (AMKD), IgA nephropathy (IgAN), primary membranous nephropathy (pMN) and other B-cell-mediated diseases.
Many of these candidates represent multibillion-dollar opportunities. Five of these programs are in pivotal development, including suzetrigine in diabetic peripheral neuropathy (DPN), a form of peripheral neuropathic pain, povetacicept for IgAN and pMN, zimislecel (VX-880) for type I diabetes and Inaxaplin in AMKD.
Enrollment has been completed in the phase III study on povetacicept for IgAN. Vertex plans to submit the first module of povetacicept’s biologics license application (BLA) for IgAN to the FDA before the end of 2025 for a potential U.S. accelerated approval. The BLA filing is expected to be completed in the first half of 2026, and Vertex expects an expedited priority review in the United States.
Some other phase III programs are also on track to complete enrollment this year, setting the stage for several potential regulatory filings in 2026 and early 2027 and potential new drug approvals in a couple of years.
On the flip side, Vertex’s dependence on just the CF franchise for revenues is a concern. CF sales are slightly slowing down. Recent pipeline setbacks in the pain program are a concern.
Vertex currently carries a Zacks Rank #3 (Hold).

Vertex Pharmaceuticals Incorporated price-consensus-chart | Vertex Pharmaceuticals Incorporated Quote
Some better-ranked stocks in the biotech sector are ANI Pharmaceuticals ANIP and Intellia Therapeutics NTLA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for ANI Pharmaceuticals’ earnings per share have increased from $7.25 to $7.29 for 2025. During the same time, earnings per share estimates for 2026 have increased from $7.74 to $7.81. Year to date, shares of ANIP have surged 70.2%.
ANI Pharmaceuticals' earnings beat estimates in each of the trailing four quarters, the average surprise being 22.66%.
In the past 60 days, estimates for Intellia’s loss per share have narrowed from $4.15 to $4.11 for 2025. During the same time, loss per share estimates for 2026 have narrowed from $4.12 to $4.07. Year to date, shares of NTLA have gained 12.1%.
Intellia’s earnings beat estimates in each of the trailing four quarters, the average surprise being 6.21%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 6 hours | |
| 11 hours | |
| 12 hours | |
| Feb-23 | |
| Feb-23 | |
| Feb-23 | |
| Feb-23 | |
| Feb-23 | |
| Feb-20 | |
| Feb-20 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-17 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite