|
|
|

|
|||||

|
|
New Feature: See Wall Street analyst ratings directly on Finviz charts for deeper context into price action.
Vertex Pharmaceuticals Incorporated’s VRTX third-quarter 2025 results were strong as it beat estimates for both earnings and sales.
The company’s total revenues of $3.08 billion rose 11% year over year, driven by higher sales of its triple combination cystic fibrosis (CF) therapy, Trikafta/Kaftrio, and contributions from its three new drugs, Alyftrek, Journavx and Casgevy. While sales rose 15% in the United States, in outside U.S. markets, sales rose 4%. Trikafta sales rose 2.6% in the quarter.
Vertex tightened its total revenue guidance for full-year 2025 from a range of $11.85-$12 billion to $11.9 to $12.0 billion.
Though Vertex revenues were primarily driven by the strong performance of its CF franchise, investor focus is more on the performance of its newer drugs, Alyftrek, Journavx and Casgevy, which hold the key to long-term growth.
Alyftrek, a once-a-day oral triple combination regimen for CF, was approved in the United States in December 2024 and in the EU in July 2025. Journavx, a novel non-opioid pain medicine (suzetrigine), was approved in the United States in January. Vertex and partner CRISPR Therapeutics’ CRSP one-shot gene therapy, Casgevy, was approved for two blood disorders, sickle cell disease and transfusion-dependent beta-thalassemia, in multiple regions in late 2023/early 2024. Vertex leads the global development and commercialization of Casgevy under the terms of the 2021 agreement with support from CRISPR Therapeutics.
While sales from Alyftrek were decent in the third quarter, Journavx and Casgevy’s sales missed expectations.
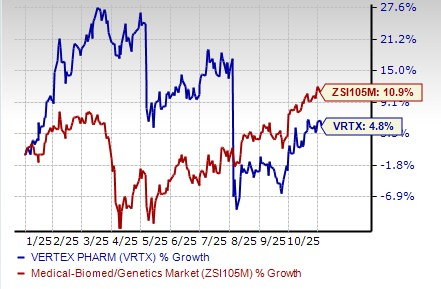
Year to date, shares of Vertex have risen 4.8% compared with the industry’s increase of 10.9%.
Let’s dig deeper to understand how these new products performed in the third quarter and the company’s outlook for the same through the rest of the year.
Alyftrek generated sales worth $247.0 million in the third quarter compared with $156.8 million in the second quarter. Vertex said that the U.S. launch of Alyftrek is progressing well across all patient groups, while in ex-U.S. markets, the early launch of Alyftrek is off to a strong start in multiple European countries where patients have reimbursed access. Alyftrek sales slightly beat the Zacks Consensus Estimate of $246 million and our model estimate of $225 million.
Vertex believes Alyftrek provides further improvements in CFTR function than Trikafta, as measured by sweat chloride. It is indicated for additional rare mutations and offers the convenience of once-daily dosing, thus showing the potential to become a standard of care for CF.
Management said it is seeing a steady uptake from all patient groups who are eligible for treatment with Alyftrek in the United States, including treatment-naive patients, patients who had discontinued previous CFTR modulator treatment, and those switching from Trikafta. Vertex expects that the majority of patients globally who are currently on Trikafta will switch to Alyftrek over time, as it offers several benefits.
Journavx (suzetrigine) generated $19.6 million in sales in the third quarter compared with $12 million in the second quarter. Journavx’s sales were almost in line with our model estimate of $20 million.
Though Journavx’s sales did not improve as expected in the third quarter. Journavx’s launch metrics and early reimbursement progress look promising. Since its launch in mid-March to mid-October, over 300,000 prescriptions have been written for Journavx across both hospital and retail settings.
Vertex said that physicians and patients have reported positive experiences with the drug while the company is making rapid contracting and formulary progress. It has reached agreements with two of the three large national PBMs to make Journavx available to their customers and is working on striking a deal with the third. Vertex also has unrestricted access within 19 state Medicaid plans. Overall, Vertex expects coverage across commercial, Medicare and Medicaid payers to continue to expand through the rest of 2025 and in 2026.
Vertex expects higher sales from Journavx in the fourth quarter as prescription volumes are rising.
Vertex and partner CRISPR’s one-shot gene therapy, Casgevy, generated sales of $16.9 million in the third quarter, down 44.4% on a sequential basis. Casgevy sales also fell short of our model estimate of $35 million.
Vertex said that more than 160 patients have initiated cell collection since launch, and 39 patients have received infusions of Casgevy. Vertex is also making rapid progress in the drug’s access and reimbursement. Vertex expects over $100 million in Casgevy revenues this year and significant growth in 2026. Vertex recorded $61.5 million in Casgevy revenues in the first nine months of 2025.
While access progress and launch metrics for both Journavx and Casgevy are promising, the revenue misses raise some concern. The drugs may take time to make a meaningful contribution to the top line.
Vertex currently carries a Zacks Rank #3 (Hold).

Vertex Pharmaceuticals Incorporated price-consensus-chart | Vertex Pharmaceuticals Incorporated Quote
Some better-ranked stocks in the biotech sector are ANI Pharmaceuticals ANIP and Intellia Therapeutics NTLA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for ANI Pharmaceuticals’ earnings per share have increased from $7.25 to $7.29 for 2025. During the same time, earnings per share estimates for 2026 have increased from $7.74 to $7.81. Year to date, shares of ANIP have surged 71.1%.
ANI Pharmaceuticals' earnings beat estimates in each of the trailing four quarters, the average surprise being 22.66%.
In the past 60 days, estimates for Intellia’s loss per share have narrowed from $4.15 to $4.11 for 2025. During the same time, loss per share estimates for 2026 have narrowed from $4.12 to $4.07. Year to date, shares of NTLA have gained 4.2%.
Intellia’s earnings beat estimates in each of the trailing four quarters, the average surprise being 6.21%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-20 | |
| Feb-20 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-19 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-17 | |
| Feb-16 | |
| Feb-15 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite