|
|
|

|
|||||

|
|
Regeneron Pharmaceuticals, Inc. REGN announced a collaboration agreement with private company Tessera Therapeutics, Inc. for the latter’s TSRA-196.
Both companies will jointly develop Tessera’s lead pipeline candidate, TSRA-196, an investigational in vivo gene editing therapy for Alpha-1 Antitrypsin Deficiency (AATD).
AATD is an inherited monogenic disease that primarily affects the lungs, liver, or both organs, affecting approximately 200,000 people in the United States and Europe.
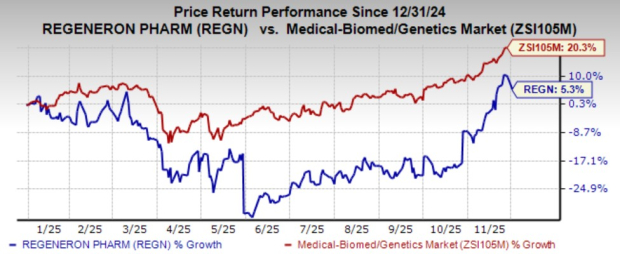
REGN stock has gained 5.3% year to date compared with the industry’s growth of 20.3%.
The collaboration is aimed at leveraging Regeneron’s long-standing expertise in genetics, genetic medicines and clinical development with Tessera’s pioneering Gene Writing and non-viral delivery platforms.
Per the terms of the agreement, Regeneron and Tessera will share worldwide development costs and future profits relating to TSRA-196 equally.
Tessera will receive a total of $150 million from Regeneron, consisting of a cash upfront payment and an equity investment. The company will also earn up to an additional $125 million in near and mid-term development milestone payments.
Tessera will oversee the initial first-in-human clinical trial after which Regeneron will assume responsibility for global development and commercialization efforts.
TSRA-196 is a potential one-time treatment to precisely correct the genetic mutation underlying AATD.
Tessera expects to file an investigational new drug and multiple clinical trial applications for TSRA-196 with the FDA by this year’s end.
The collaboration follows the positive data presented by Tessera at the American Society of Gene & Cell Therapy 28th Annual Meeting.
Tessera presented preclinical data that highlighted durable, high-fidelity genome editing of SERPINA1, the locus responsible for AATD, in mice and non-human primates following a single dose of TSRA-196, with high liver editing specificity, no germline or off-target editing, and favorable safety and tolerability using Tessera’s proprietary lipid nanoparticle delivery vehicle.
These data also reinforce TSRA-196’s potential to correct the underlying genetic cause of AATD and support its advancement into clinical development.
REGN’s performance over the past month is impressive on the back of positive regulatory updates.
Regeneron recently announced that the FDA has approved Eylea HD (aflibercept) Injection 8 mg for the treatment of patients with macular edema following retinal vein occlusion (RVO) with up to every eight-week dosing after an initial monthly dosing period.
Regeneron’s lead drug Eylea is an anti-vascular endothelial growth factor inhibitor (VEGF). The drug, approved for various ophthalmology indications, is a major contributor to the top line.
However, Eylea is under pressure due to competition from Roche’s RHHBY Vabysmo.
Since Eylea accounts for a majority of REGN’s sales, a rapid decline in sales has adversely impacted its top line.
To counter the decline in Eylea sales, Regeneron has developed a higher dose of the drug, Eylea HD.
The regulatory body also approved a monthly dosing option for some patients who may benefit from resuming this dosing schedule across approved indications — wet age-related macular degeneration (wAMD), diabetic macular edema (DME), diabetic retinopathy (DR) and RVO.
The European Commission (EC) recently approved a label expansion of Libtayo as an adjuvant treatment for adult patients with CSCC at high risk of recurrence after surgery and radiation.
The recent label expansion by EC expands the existing approved indication for Libtayo in advanced CSCC to include patients at high risk of disease recurrence.
The drug was also approved by the FDA in October for the same indication.
REGN’s progress with the oncology portfolio should enable diversification of top-line growth.
REGN’s top line also comprises its share of profits/losses in connection with the global sales of Dupixent. Partner Sanofi records global net product sales of Dupixent. Consistent label expansions of Dupixent continue to fuel its sales and help REGN earn higher profits.
However, the decline in Eylea sales remains a headwind as it was the growth engine for REGN.
Regeneron currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are CorMedix CRMD and ANI Pharmaceuticals ANIP, both currently sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for CorMedix’s 2025 earnings per share (EPS) have increased from $1.83 to $2.87. EPS estimates for 2026 have moved up from $2.48 to $2.88 during the same period. CRMD stock has surged 20.8% year to date.
CorMedix’s earnings beat estimates in each of the trailing four quarters, with an average surprise of 27.04%.
In the past 60 days, estimates for ANI Pharmaceuticals’ earnings per share have increased from $7.28 to $7.29 for 2025. During the same time, earnings per share estimates for 2026 have increased from $7.78 to $7.81. Year to date, shares of ANIP have surged 52.8%.
ANI Pharmaceuticals' earnings beat estimates in each of the trailing four quarters, the average surprise being 21.24%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-12 | |
| Feb-12 | |
| Feb-12 | |
| Feb-12 | |
| Feb-12 | |
| Feb-12 | |
| Feb-11 | |
| Feb-11 | |
| Feb-11 | |
| Feb-11 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite