|
|
|

|
|||||

|
|
This was a low-key week for the biotech sector before the onset of the first-quarter earnings season. Biotech giant Bristol Myers Squibb BMY was down on data from the schizophrenia study while Regeneron REGN was in the spotlight on updates on key drugs.
Bristol Myers announced disappointing top-line results from the phase III ARISE study on schizophrenia drug Cobenfy (xanomeline and trospium chloride). The study is evaluating the efficacy and safety of Cobenfy as an adjunctive treatment to atypical antipsychotics in adults with inadequately controlled symptoms of schizophrenia.
Treatment with Cobenfy as an adjunctive demonstrated a 2.0-point reduction in the Positive and Negative Syndrome Scale total score compared to placebo with an atypical antipsychotic at week six. However, this data did not reach the threshold for statistical significance for the primary endpoint. Shares of BMY were down following the data announcement.
Preliminary analyses suggest that Cobenfy as an adjunctive treatment to an atypical antipsychotic was associated with improvements in symptoms of schizophrenia compared to placebo plus an atypical antipsychotic for certain patients.
Last week, BMY announced that the FDA updated the label for the heart disease drug Camzyos. The label updates include simplified twice-yearly echo monitoring for eligible CAMZYOS patients in the maintenance phase and expanded patient eligibility with reduced contraindications.
Regeneron announced a significant expansion of its manufacturing capacity in the United States through a new agreement with FUJIFILM Diosynth Biotechnologies. Per the agreement, Regeneron will nearly double its large-scale manufacturing capacity in the United States by accessing Fujifilm’s new state-of-the-art biopharmaceutical facility. The total investment is estimated to exceed $3 billion.
Last week, Regeneron announced that the FDA has issued a complete response letter (CRL) to the company’s supplemental biologics license application (sBLA) seeking addition of extended dosing intervals (up to every 24 weeks) for Eylea HD (aflibercept) Injection 8 mg across all approved indications.
While the CRL did not identify any issue with the safety or efficacy of EYLEA HD in its approved indications and dosing regimens, the FDA did not agree with Regeneron’s proposal to add additional extended dosing intervals (greater than every 16 weeks, which is the maximum dosing interval currently indicated in the label).
The regulatory body accepted Regeneron’s sBLA seeking approval of Eylea HD for both the treatment of macular edema following retinal vein occlusion, and for broadening the dosing schedule to include every four-week (monthly) dosing across approved indications. The target action date is Aug. 19, 2025, following the use of a Priority Review voucher.
Regeneron and partner Sanofi SNY obtained FDA approval for yet another label expansion of asthma drug Dupixent (dupilumab). The drug is now approved in the United States for the treatment of adults and adolescents aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite histamine-1 (H1) antihistamine treatment. CSU is the seventh disease with underlying type 2 inflammation for which Dupixent is approved.
Regeneron and Sanofi are studying dupilumab in a broad range of diseases driven by type 2 inflammation or other allergic processes in phase III studies, including chronic pruritus of unknown origin, bullous pemphigoid and lichen simplex chronicus.
Gilead Sciences, Inc. GILD announced positive top-line results from the phase III ASCENT-04/KEYNOTE-D19 study on breast cancer drug Trodelvy (sacituzumab govitecan-hziy).
Data showed that Trodelvy, in combination with Keytruda, significantly improved progression-free survival (PFS) compared to Keytruda and chemotherapy in patients with inoperable (unresectable) locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors express PD-L1 (CPS ≥ 10). The study met its primary endpoint, showing a statistically significant and clinically meaningful improvement in PFS. Overall survival (OS) is a key secondary endpoint and was not mature at the time of the PFS primary analysis.
Trodelvy plus Keytruda showed an early trend in improving OS versus the standard of care in patients with previously untreated PD-L1+ (CPS ≥10) mTNBC.
Gilead is evaluating Trodelvy in the ASCENT-03 pivotal trial in first-line setting in mTNBC patients who are not candidates for PD-L1 based therapy, the ASCENT-05 pivotal trial in patients with early-stage TNBC (eTNBC), and the ASCENT-07 pivotal trial in patients with HR+/HER2- mBC who have received endocrine therapy. Trodelvy is also being investigated in additional phase III studies in other disease settings, including lung and gynecological cancers.
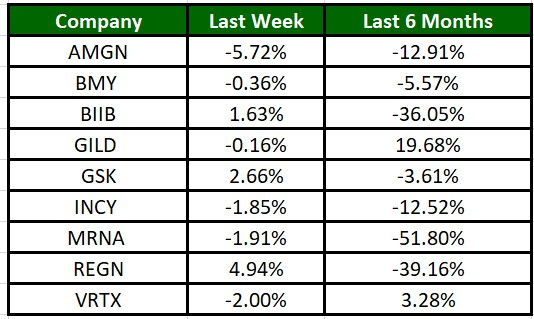
The Nasdaq Biotechnology Index has gained 0.94% in the past four trading sessions and AMGN’s shares have lost 5.72%. In the past six months, shares of MRNA have plunged 51.8%. (See the last biotech stock roundup here: Biotech Stock Roundup: BMY Down on Study Failure, VERV Up on Study Data & More News)
Stay tuned for earnings updates along with regular pipeline updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 3 hours | |
| 9 hours | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-13 | |
| Feb-12 | |
| Feb-12 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite