|
|
|

|
|||||

|
|
Alnylam Pharmaceuticals ALNY reported first-quarter 2025 adjusted loss of 1 cent per share, narrower than the Zacks Consensus Estimate of a loss of 56 cents. The company had incurred a loss of 16 cents per share in the year-ago quarter. The adjusted figure excluded items like stock-based compensation expenses and unrealized loss on marketable equity securities.
Alnylam recorded total revenues of $594.2 million in the quarter, which surpassed the Zacks Consensus Estimate of $588.2 million. In the year-ago quarter, total revenues were $494.3 million. The top line rose 20% year over year on a reported basis and 22% at a constant exchange rate (CER), mainly driven by increased product sales. (See the Zacks Earnings Calendar to stay ahead of market-making news.)
Net product revenues were $468.5 million, up 28% year over year on a reported basis and 30% at CER, driven by strong growth in patient demand for the newly approved drug, Amvuttra (vutrisiran), as well as for Givlaari (givosiran) and Oxlumo (lumasiran).
Net revenues from collaborators were $99.2 million, down 16% from the year-ago quarter, primarily due to a $65 million milestone payment received from Roche RHHBY in the year-ago quarter. Alnylam also recognizes revenues under its ongoing collaborations with Regeneron REGN and Novartis NVS.
Despite the beat, shares of the company are down 3% on Thursday, likely because investors expected revenues to surpass expectations by a higher margin.
Onpattro (patisiran) is approved for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis. The injection recorded sales of $49.5 million in the reported quarter, down 29% on a reported basis. Onpattro sales missed the Zacks Consensus Estimate of $51.2 million as well as our model estimate of $52.7 million.
Amvuttra is FDA-approved for the treatment of adult patients with polyneuropathy of hATTR amyloidosis. The European Commission also approved Amvuttra for treating hATTR amyloidosis in adult patients with stage 1 or 2 polyneuropathy. Recently, the drug has also been approved by the FDA for treating cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality, cardiovascular hospitalizations and urgent heart failure visits. A regulatory application seeking approval of Amvuttra for the ATTR-CM indication in the EU is currently under review.
Amvuttra generated sales worth $310 million in the first quarter, up 59% on a reported basis. The uptake for the product has been encouraging, with new patients starting treatment as well as several patients switching from Onpattro. The expanded label is also expected to boost sales in the quarters ahead. Amvuttra sales beat the Zacks Consensus Estimate of $300 million as well as our model estimate of $275.8 million.
Givlaari, approved for the treatment of acute hepatic porphyria, recorded sales of $67 million, reflecting a year-over-year increase of 15% on a reported basis. Givlaari sales marginally missed the Zacks Consensus Estimate of $67.5 million and our model estimate of $68.7 million. Oxlumo recorded global net product revenues of $42.1 million in the reported quarter, which was relatively flat year over year on a reported basis. However, Oxlumo sales missed the Zacks Consensus Estimate of $44.7 million as well as our estimate of $43.7 million.
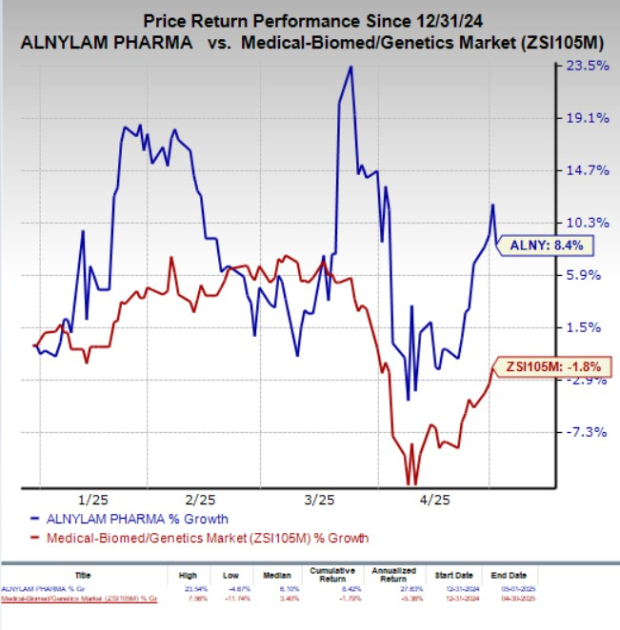
Year to date, ALNY stock has gained 8.4% against the industry’s 1.8% decline.
Adjusted research and development (R&D) expenses were mostly flat year over year at $241.3 million. R&D expenses accounted for costs associated with the KARDIA-3 clinical study evaluating zilebesiran to treat patients with hypertension at high cardiovascular risk, in partnership with Roche. Pre-clinical activities and expenses associated with mivelsiran cAPPRicorn-1 clinical studies also contributed to R&D costs. However, such costs were offset by reductions related to the wind-down of the phase III HELIOS-B study of Amvuttra for the ATTR-CM indication.
Adjusted selling, general and administrative (SG&A) expenses increased 12% year over year to $207 million, mainly due to ramped-up marketing activities for the promotion of Amvuttra for the cardiomyopathy launch and increased employee compensation expenses.
Cash, cash equivalents and marketable securities as of March 31, 2025, amounted to $2.63 billion compared with $2.69 billion recorded as of Dec. 31, 2024, with the decrease primarily due to operating activities.
Alnylam continues to expect net product revenues for Onpattro, Amvuttra, Givlaari and Oxlumo in the range of $2.05-$2.25 billion for 2025.
Net revenues from collaborations and royalties are expected in the range of $650-$750 million. Adjusted R&D and SG&A expenses are anticipated in the band of $2.1-$2.2 billion.
Alnylam, in collaboration with Regeneron, is advancing cemdisiran, an investigational RNAi therapeutic for the treatment of complement-mediated diseases. The agreement regarding cemdisiran underwent a modification last year. Per the modified agreement, Alnylam granted exclusive rights to Regeneron to develop cemdisiran as monotherapy and in combination with anti-C5 antibodies for complement-mediated indications.
ALNY regained full global development and commercialization rights to mivelsiran in all indications in 2024, after Regeneron opted out of further co-development and co-commercialization of mivelsiran in development for cerebral amyloid angiopathy and Alzheimer's disease. However, Regeneron will be eligible to receive low double-digit royalties on sales of mivelsiran, if approved.
Alnylam, in collaboration with Roche, is developing zilebesiran in a mid-stage study to treat hypertension.
Alnylam has granted Novartis exclusive global rights to manufacture and commercialize RNAi therapeutics targeting PCSK9, including Leqvio (inclisiran), for hypercholesterolemia and other diseases. The FDA has approved Leqvio for earlier use in high-risk patients alongside diet and statins. As of April 2025, Leqvio is approved in more than 100 countries, with ongoing late-stage studies for further label expansion.

Alnylam Pharmaceuticals, Inc. price-consensus-chart | Alnylam Pharmaceuticals, Inc. Quote
Alnylam reported better-than-expected results in the first quarter of 2025, beating earnings and revenue estimates. The year-over-year increase in revenues has been mainly fueled by growing Amvuttra sales as a result of increased patient demand. The recent label expansion of the drug for the ATTR-CM indication in the United States has further expanded the eligible patient population for the drug, which is expected to boost further sales in the quarters ahead. A nod in the EU for Amvuttra’s new indication is also expected soon. Sales of other products, Givlaari and Oxlumo, are also contributing to the top line.
Additionally, Alnylam’s ongoing collaboration agreements with several large biotech firms, developing candidates for various indications, are encouraging. Successful development and potential approval of these candidates will further expand the company’s commercial portfolio. ALNY also earns collaboration revenues from its partners, which boost the top line. Thus, we believe that Alnylam has significant potential to boost shareholder value in the long term.
Alnylam currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
This article originally published on Zacks Investment Research (zacks.com).
| 3 hours | |
| 4 hours | |
| Feb-26 | |
| Feb-26 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 | |
| Feb-25 |
Slate Medicines raises $130m in funds to progress anti-PACAP treatments
NVS
Pharmaceutical Technology
|
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-24 | |
| Feb-23 | |
| Feb-23 | |
| Feb-23 |
Join thousands of traders who make more informed decisions with our premium features. Real-time quotes, advanced visualizations, backtesting, and much more.
Learn more about FINVIZ*Elite